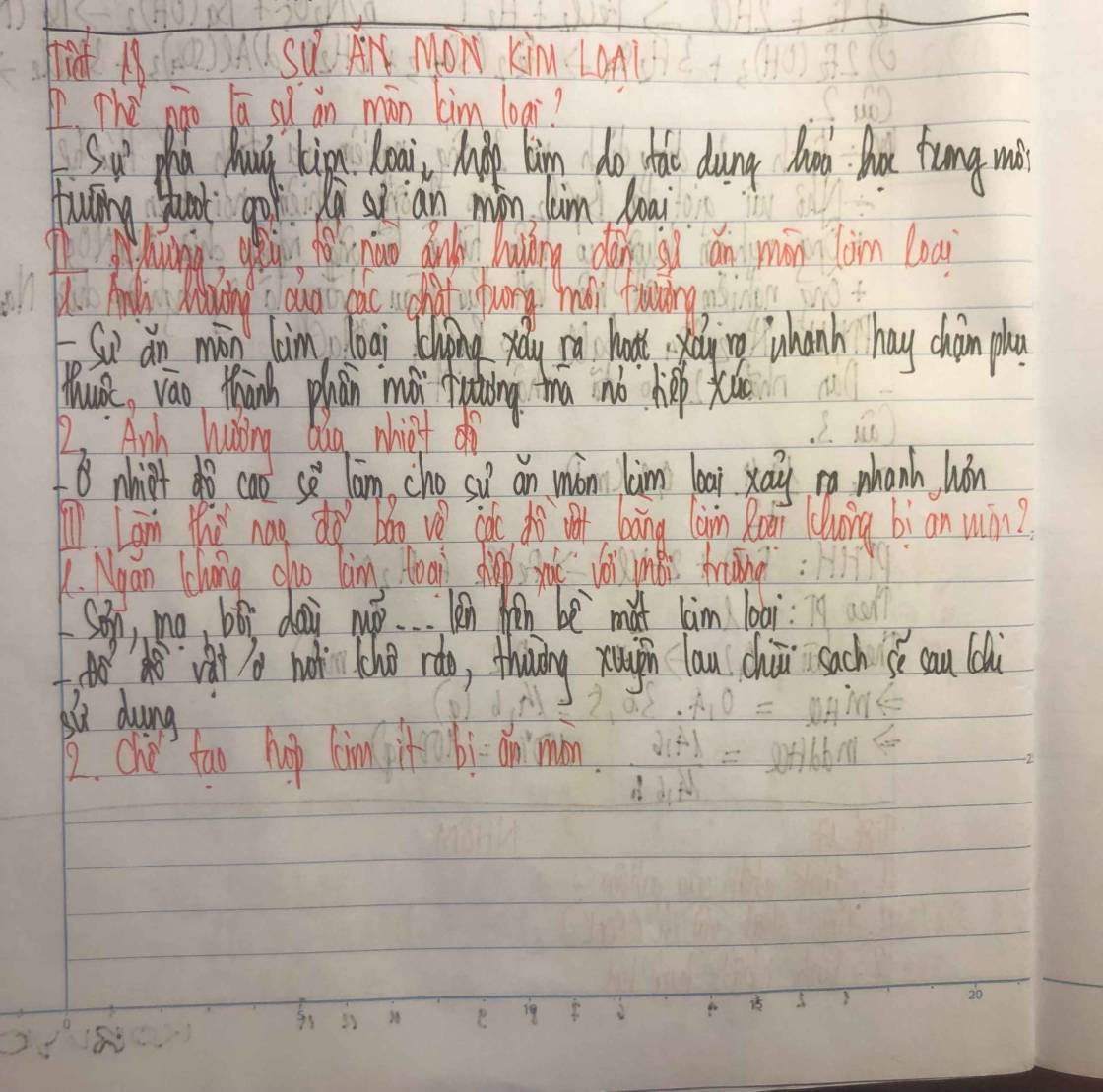
Đào Thị Quỳnh Chi 9A nôp bài ạ
Đào Thị Quỳnh Chi 9A nôp bài ạ
Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.



Các chất td được với dd H2SO4 loãng là: CuO, Cu(OH)2, Na2SO3, MgSO3, Al,Fe, Al(OH)3, Fe2O3, Fe3O4
\(CuO+H_2SO_4\rightarrow CuSO_4+H_2O\\ 2Al+3H_2SO_4\rightarrow Al_2\left(SO_4\right)_3+3H_2\\ Fe+H_2SO_4\rightarrow FeSO_4+H_2\\ 2Al\left(OH\right)_3+3H_2SO_4\rightarrow Al_2\left(SO_4\right)_3+6H_2O\\ Fe_2O_3+3H_2SO_4\rightarrow Fe_2\left(SO_4\right)_3+3H_2O\\ Fe_3O_4+4H_2SO_4\rightarrow FeSO_4+Fe_2\left(SO_4\right)_3+4H_2O\\ Na_2SO_3+H_2SO_4\rightarrow Na_2SO_4+SO_2+H_2O\\ Cu\left(OH\right)_2+H_2SO_4\rightarrow CuSO_4+2H_2O\\ MgSO_3+H_2SO_4\rightarrow MgSO_4+SO_2+H_2O\)

Ta có: \(n_{H_2}=\dfrac{5,6}{22,4}=0,25\left(mol\right)\)
PT: \(Fe+2HCl\rightarrow FeCl_2+H_2\)
Theo PT: \(n_{Fe}=n_{H_2}=0,25\left(mol\right)\Rightarrow m_{Fe}=0,25.56=14\left(g\right)=m_1\)
\(n_{HCl}=2n_{H_2}=0,5\left(mol\right)\)
\(\Rightarrow m_{HCl}=0,5.36,5=18,25\left(g\right)\)
\(\Rightarrow m_{ddHCl}=\dfrac{18,25}{7,3\%}=250\left(g\right)=m_2\)

a, \(2NaOH+H_2SO_4\rightarrow Na_2SO_4+2H_2O\)
\(2KOH+H_2SO_4\rightarrow K_2SO_4+2H_2O\)
Ta có: 40nNaOH + 56nKOH = 25,44 (1)
\(n_{H_2SO_4}=\dfrac{1}{2}n_{NaOH}+\dfrac{1}{2}n_{KOH}=0,3.0,9=0,27\left(mol\right)\left(2\right)\)
Từ (1) và (2) \(\Rightarrow\left\{{}\begin{matrix}n_{NaOH}=0,3\left(mol\right)\\n_{KOH}=0,24\left(mol\right)\end{matrix}\right.\)
\(\Rightarrow\left\{{}\begin{matrix}m_{NaOH}=0,3.40=12\left(g\right)\\m_{KOH}=0,24.56=13,44\left(g\right)\end{matrix}\right.\)
b, \(m_{ddH_2SO_4}=300.1,14=342\left(g\right)\)
\(\Rightarrow C\%_{H_2SO_4}=\dfrac{0,27.98}{342}.100\%\approx7,74\%\)

\(n_{CuO}=\dfrac{8}{80}=0,1\left(mol\right)\)
\(m_{HCl}=73.20\%=14,6\left(g\right)\Rightarrow n_{HCl}=\dfrac{14,6}{36,5}=0,4\left(mol\right)\)
PT: \(CuO+2HCl\rightarrow CuCl_2+H_2O\)
Xét tỉ lệ: \(\dfrac{0,1}{1}< \dfrac{0,4}{2}\), ta được HCl dư.
Theo PT: \(n_{CuCl_2}=n_{CuO}=0,1\left(mol\right)\)
\(\Rightarrow m_{CuCl_2}=0,1.135=13,5\left(g\right)\)

\(a)n_{H_2}=\dfrac{7,55}{22,4}=\dfrac{151}{448}mol\\ n_{Mg}=n_{Zn}=a;n_{Fe}=c\\ Mg+H_2SO_4\rightarrow MgSO_4+H_2\\ a.....a\\ Zn+H_2SO_4\rightarrow ZnSO_4+H_2\\ a.....a\\ Fe+H_2SO_4\rightarrow FeSO_4+H_2\\ b.....b\\ \Rightarrow\left\{{}\begin{matrix}24a+65a+56b=16\\2a+b=\dfrac{151}{448}\end{matrix}\right.\\ \Rightarrow a=0,125;b=\dfrac{39}{448}\\ \%m_{Mg}=\dfrac{24.0,125}{16}\cdot100=18,75\%\\ \%m_{Zn}=\dfrac{65.0,125}{16}\cdot100=50,78\%\\ \%m_{Fe}=100-18,75-50,78=30,47\%\\ b)V_{ddH_2SO_4}=\dfrac{0,125.2+\dfrac{39}{448}}{1}\approx0,337l\)