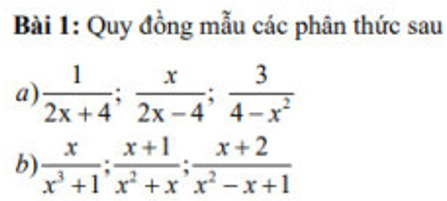 chình bày đầy đủ luôn nha
chình bày đầy đủ luôn nha
Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.


Gọi hóa trị của Cu,Fe,S,Ba lần lượt là a,b,c,d>0
\(a,Cu_2^aO_1^{II}\Rightarrow a\cdot2=1\cdot II\Rightarrow a=1\Rightarrow Cu\left(I\right)\\ Cu_1^aO_1^{II}\Rightarrow a\cdot1=II\cdot1\Rightarrow a=2\Rightarrow Cu\left(II\right)\\ b,Fe_1^bO_1^{II}\Rightarrow b\cdot I=II\cdot1\Rightarrow b=2\Rightarrow Fe\left(II\right)\\ Fe_2^bO_3^{II}\Rightarrow2b=II\cdot3\Rightarrow b=3\Rightarrow Fe\left(III\right)\\ c,S_1^cO_2^{II}\Rightarrow c=II\cdot2=4\Rightarrow S\left(IV\right)\\ S_1^cO_3^{II}\Rightarrow c=3\cdot II=6\Rightarrow S\left(VI\right)\\ H_2^IS_1^c\Rightarrow c=I\cdot2=2\Rightarrow S\left(II\right)\\ d,Ba_1^d\left(CO_3\right)_1^{II}\Rightarrow d=II\cdot1=2\Rightarrow Ba\left(II\right)\)
gọi hóa trị của các nguyên tố cần tìm là \(x\)
a/
\(\rightarrow Cu_2^xO^{II}_1\rightarrow x.2=II.1\rightarrow x=I\)
vậy Cu hóa trị I
\(\rightarrow Cu^x_1O^{II}_1\rightarrow x.1=II.1\rightarrow x=II\)
vậy Cu hóa trị II
b/
\(\rightarrow Fe^x_1O_1^{II}\rightarrow x.1=II.1\rightarrow x=II\)
vậy Fe hóa trị II
\(\rightarrow Fe_2^xO^{II}_3\rightarrow x.2=II.3\rightarrow x=III\)
vậy Fe hóa trị III
c/
\(\rightarrow S^x_1O_2^{II}\rightarrow x.1=II.2\rightarrow x=IV\)
vậy S hóa trị IV
\(\rightarrow S^x_1O_3^{II}\rightarrow x.1=II.3\rightarrow x=VI\)
vậy S hóa trị VI
\(\rightarrow H^I_2S^x_1\rightarrow I.2=x.1\rightarrow x=II\)
vậy S hóa trị II
d/ \(\rightarrow Ba^x_1\left(CO_3\right)^{II}_1\rightarrow x.1=II.1\rightarrow x=II\)
vậy Ba hóa trị II

\(a,CTTQ:Mg_x^{II}\left(OH\right)_y^I\\ \Rightarrow x\cdot II=y\cdot I\Rightarrow\dfrac{x}{y}=\dfrac{1}{2}\Rightarrow x=1;y=2\\ \Rightarrow Mg\left(OH\right)_2\\ b,CTTQ:Al_x^{III}\left(SO_4\right)_y^{II}\\ \Rightarrow x\cdot III=y\cdot II\Rightarrow\dfrac{x}{y}=\dfrac{2}{3}\Rightarrow x=2;y=3\\ \Rightarrow Al_2\left(SO_4\right)_3\\ c,CTTQ:Fe_x^{III}O_y^{II}\\ \Rightarrow x\cdot III=y\cdot II\Rightarrow\dfrac{x}{y}=\dfrac{2}{3}\Rightarrow x=2;y=3\\ \Rightarrow Fe_2O_3\\ d,CTTQ:Cu_x^{II}\left(CO_3\right)_y^{II}\\ \Rightarrow x\cdot II=y\cdot II\Rightarrow\dfrac{x}{y}=1\Rightarrow x=1;y=1\\ \Rightarrow CuCO_3\)
\(e,CTTQ:Na_x^I\left(PO_4\right)_y^{III}\\ \Rightarrow x\cdot I=y\cdot III\Rightarrow\dfrac{x}{y}=3\Rightarrow x=3;y=1\\ \Rightarrow Na_3PO_4\\ f,CTTQ:Ca_x^{II}\left(NO_3\right)_y^I\\ \Rightarrow x\cdot II=y\cdot I\Rightarrow\dfrac{x}{y}=\dfrac{1}{2}\Rightarrow x=1;y=2\\ \Rightarrow Ca\left(NO_3\right)_2\)
\(a,PTK_{Mg\left(OH\right)_2}=24+17\cdot2=61\left(đvC\right)\\ b,PTK_{Al_2\left(SO_4\right)_3}=27\cdot2+\left(32+16\cdot4\right)\cdot3=342\left(đvC\right)\\ c,PTK_{Fe_2O_3}=56\cdot2+16\cdot3=160\left(đvC\right)\\ d,PTK_{CuCO_3}=64+12+16\cdot3=124\left(đvC\right)\\ e,PTK_{Na_3PO_4}=23\cdot3+31+16\cdot4=164\left(đvC\right)\\ f,PTK_{Ca\left(NO_3\right)_2}=40+\left(14+16\cdot3\right)\cdot2=164\left(đvC\right)\)


bạn đăng tách cho mn giúp nhé
Bài 6 :
\(\Rightarrow30-3y=xy\Leftrightarrow xy+3y=30\Leftrightarrow y\left(x+3\right)=30\)
\(\Rightarrow x+3;y\inƯ\left(30\right)=\left\{\pm1;\pm2;\pm3;\pm5;\pm6;\pm10;\pm15;\pm30\right\}\)
| x + 3 | 1 | -1 | 2 | -2 | 3 | -3 | 5 | -5 | 6 | -6 | 10 | -10 | 15 | -15 | 30 | -30 |
| y | 30 | -30 | 15 | -15 | 10 | -10 | 6 | -6 | 5 | -5 | 3 | -3 | 2 | -2 | 1 | -1 |
| x | -2 | -4 | -1 | -5 | 0 | -6 | 2 | -8 | 3 | -9 | 7 | -13 | 12 | -18 | 27 | -33 |

= 1/1x2 + 1/2x3 + 1/3x4 + 1/4x5 + ...... + 1/9x10
= 1/1 - 1/2 + 1/2 - 1/3 + 1/3 - 1/4 + 1/4-1/5 +.......+ 1/9 -1/10
= 1/1 - 1/10
= 9/10

Thế tích hình hộp chữ nhật là:
20x16x10=3200(cm2)
Đáp số: 3200 cm2.

\(a,\dfrac{1}{2x+4}=\dfrac{x-2}{2\left(x+2\right)\left(x-2\right)};\dfrac{x}{2x-4}=\dfrac{x\left(x+2\right)}{2\left(x-2\right)\left(x+2\right)}\\ \dfrac{3}{4-x^2}=\dfrac{-6}{2\left(x-2\right)\left(x+2\right)}\\ b,\dfrac{x}{x^3+1}=\dfrac{x^2}{x\left(x+1\right)\left(x^2-x+1\right)}\\ \dfrac{x+1}{x^2+x}=\dfrac{\left(x+1\right)\left(x^2-x+1\right)}{x\left(x+1\right)\left(x^2-x+1\right)}\\ \dfrac{x+2}{x^2-x+1}=\dfrac{x\left(x+1\right)\left(x+2\right)}{x\left(x+1\right)\left(x^2-x+1\right)}\)