thể tích khí oxi sinh ra (đktc) khi nung 0,4mol KMnO4 là
Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.


a) \(n_{KMnO_4}=\dfrac{15,8}{158}=0,1\left(mol\right)\)
\(PTHH:2KMnO_4\underrightarrow{t^o}K_2MnO_4+MnO_2+O_2\uparrow\)
(mol)..........0,1................0,05..........0,05......0,05
\(V_{O_2}=0,05.22,4=1,12\left(l\right)\)
b) \(n_{Fe}=\dfrac{1.68}{56}=0,03\left(mol\right)\)
\(PTHH:3Fe+2O_2\underrightarrow{t^o}Fe_3O_4\)
(mol).......0,03....0,02.......0,1
\(PTHH:2KMnO_4\underrightarrow{t^o}K_2MnO_4+MnO_2+O_2\uparrow\)
(mol)..........0,04..............0,02............0,02....0,02
\(m_{KMnO_4}=0,04.158=6,32\left(g\right)\)
\(m_{KMnO_4\left(thựctế\right)}=6,32:95\%\approx6,65\left(g\right)\)

\(2KMnO_4\underrightarrow{^{t^0}}K_2MnO_4+MnO_2+O_2\)
\(0.4..................................................0.2\)
\(V_{O_2}=0.2\cdot22.4=4.48\left(l\right)\)

\(PTHH:2KClO_3\underrightarrow{t^o}2KCl+3O_2\\ \left(mol\right)..0,2\rightarrow.......0,2.......0,3\\ V_{O_2}=0,3.22,4=6,72\left(l\right)\)
\(PTHH:2KMnO_4\underrightarrow{t^o}K_2MnO_4+MnO_2+O_2\\ \left(mol\right)......0,2..\rightarrow.....0,1.........0,1........0,1\\ V_{O_2}=0,1.22,4=2,24\left(l\right)\)
PTHH:2KClO3to→2KCl+3O2
.0,2→.......0,2.......0,3 mol
VO2=0,3.22,4=6,72(l)
PTHH:2KMnO4→K2MnO4+MnO2+O2
......0,2..→.....0,1.........0,1........0,1 mol
VO2=0,1.22,4=2,24(l)

\(n_{KClO_3}=\dfrac{24,5}{122,5}=0,2mol\)
a)\(2KClO_3\underrightarrow{t^o}2KCl+3O_2\)
0,2 0,3
b)\(V_{O_2}=0,3\cdot22,4=6,72l\)
c)Bảo toàn khối lượng:
\(m_{Fe}+m_{O_2}=m_{sp}\)
\(\Rightarrow m_{sp}=11,2+0,3\cdot32=20,8g\)

nKMnO4 = 7,9 : 158 = 0,05 (mol)
pthh : 2KMnO4 -t--> K2MnO4 + MnO2 + O2
0,05 0,025
=> VO2 = 0,025 . 22,4 = 0,56 (L)
nS= 2,4 : 32 = 0,075 (mol)
pthh : S + O2 -t-> SO2
LTL : 0,075 > 0,025
=> S dư
theo pthh : nO2 = nSO2 = 0,025 (mol)
=> mSO2 = 0,025 . 64 = 1,6 (G)
\(n_{KMnO_4}=\dfrac{7,9}{158}=0,05\left(mol\right)\)
PTHH: 2KMnO4 --to--> K2MnO4 + MnO2 + O2
0,05 0,025
=> VO2 = 0,025.22,4 = 0,56 (l)
nS = \(\dfrac{2,4}{32}=0,075\left(mol\right)\)
PTHH: S + O2 --to--> SO2
LTL: \(0,075>0,025\rightarrow\) S dư
\(n_{SO_2}=n_{O_2}\rightarrow m_{SO_2}=0,025.64=1,6\left(g\right)\)

a. \(n_{CH_4}=\dfrac{10.08}{22,4}=0,45\left(mol\right)\)
PTHH : CH4 + 2O2 ----to---> CO2 + 2H2O
0,45 0,9 0,45
\(V_{O_2}=0,9.22,4=20,16\left(l\right)\)
\(V_{kk}=20,16.5=100,8\left(l\right)\)
b. \(m_{CO_2}=0,45.44=19,8\left(g\right)\)
c. PTHH : 2KMnO4 -> K2MnO4 + MnO2 + O2
1,8 0,9
\(m_{KMnO_4}=1,8.158=284,4\left(g\right)\)
Bài 1:
Ta có nCH4 = 5,622,4 = 0,25 ( mol )
CH4 + 2O2 → H2O + CO2↑
0,25......0,5.......0,25....0,25
=> VO2 = 0,5 . 22,4 = 11,2 ( lít )
=> mH2O = 18 . 0,25 = 4,5 ( gam )
=> mCO2 = 0,25 . 44 = 11 ( gam )

2KClO3 -- > 2KCl + O2
nKClO3 = 73,5 / 122,5 = 0,6 (mol)
mKCl = 0,6 . 74,5 = 44,7 (g)
VO2 = 0,3 . 22,4 = 6,72 (l)
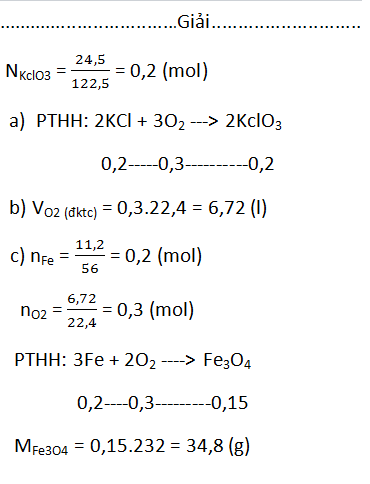
\(2KMnO_4\underrightarrow{^{t^0}}K_2MnO_4+MnO_2+O_2\)
\(0.4.................................................0.2\)
\(V_{O_2}=0.2\cdot22.4=4.48\left(l\right)\)