Dùng khí H2 để khử hoàn toàn hỗn hợp gồm PbO và CuO thu được 2,07 gam Pb và 1,6 gam Cu. Hãy tính:
a. Khối lượng hỗn hợp oxit ban đầu.
b. Tổng thể tích H2 đã dùng (đktc)
Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

PTHH:CuO+COto→Cu+CO2(1)(1)
PbO+COto→Pb+CO2(2)
Theo(1) nCuO=nCu=1,664=0,025(mol)
mCuO=0,025.80=2g
Theo(2) nPbO=nPb=\(\dfrac{2,07}{207}\)=0,01mol
mPbO=0,01.223=2,23g
b) Theo(1) và (2): ΣnCO=nCu+nPb=0,025+0,01=0,035mol
ΣVCO=0,035.22,4=0,784lit

\(CuO+CO\rightarrow Cu+CO_2\)
..x..........x.........................
\(PbO+CO\rightarrow Pb+CO_2\)
..y........y........................
- Theo bài ra ta có hệ : \(\left\{{}\begin{matrix}80x+223y=3,83\\x+y=0,03\end{matrix}\right.\)
\(\Rightarrow\left\{{}\begin{matrix}x=0,02\\y=0,01\end{matrix}\right.\)
\(\Rightarrow\left\{{}\begin{matrix}m_{CuO}=1,6\\m_{PbO}=2,23\end{matrix}\right.\) ( g )
b, \(n_K=n_{CO_2}=x+y=0,03\left(mol\right)\)
\(\Rightarrow V=0,672\left(l\right)\)
c, \(Ca\left(OH\right)_2+CO_2\rightarrow CaCO_3+H_2O\)
........................0,03........0,03.............
\(\Rightarrow m_{kt}=3\left(g\right)\)
Đặt \(\left\{{}\begin{matrix}n_{CuO}=x\left(mol\right)\\n_{PbO}=y\left(mol\right)\end{matrix}\right.\)
\(m_{CuO}+m_{PbO}=3,83\\ \Rightarrow80x+223y=3,83\left(1\right)\)
\(PTHH:CuO+CO\underrightarrow{t^o}Cu+CO_2\uparrow\\ \left(mol\right)......x\rightarrow..x....x.....x\\ PTHH:PbO+CO\underrightarrow{t^o}Pb+CO_2\uparrow\\ \left(mol\right)......y\rightarrow..y....y.....y\\ n_{CO}=\dfrac{0,84}{28}=0,03\\ \Rightarrow x+y=0,03\left(2\right)\)
Từ (1) và (2) ta có hpt \(\left\{{}\begin{matrix}80x+223y=3,83\\x+y=0,03\end{matrix}\right.\)
Giải hpt ta được \(\left\{{}\begin{matrix}x=0,02\\y=0,01\end{matrix}\right.\)
\(a,\left\{{}\begin{matrix}m_{CuO}=80.0,02=1,6\left(g\right)\\m_{PbO}=3,83-1,6=2,23\left(g\right)\end{matrix}\right.\)
\(b,V_{CO_2}=\left(x+y\right).22,4=\left(0,02+0,01\right).22,4=0,672\left(l\right)\)
\(c,n_{CO_2}=x+y=0,02+0,01=0,03\left(mol\right)\\ PTHH:Ca\left(OH\right)_2+CO_2\rightarrow CaCO_3\downarrow+H_2O\\ \left(mol\right)................0,03\rightarrow0,03\\ m_{CaCO_3}=0,03.100=3\left(g\right)\)

a, -Gọi số mol của CuO và Fe2O3 lần lượt là x, y ( mol )
PTKL : \(80x+160y=40\left(I\right)\)
\(CuO+H_2\rightarrow Cu+H_2O\)
..x.........x............
\(Fe_2O_3+3H_2\rightarrow2Fe+3H_2O\)
...y............3y......
=> \(n_{H_2}=x+3y=\dfrac{V}{22,4}=0,6\left(mol\right)\left(II\right)\)
- Giair I và II ta được : x = 0,3 , y = 0,1 ( mol )
=> \(\left\{{}\begin{matrix}mCuO=n.M=24\left(g\right)\\mFe2O3=mhh-mCuO=16\left(g\right)\end{matrix}\right.\)
b, \(\%CuO=\dfrac{m}{mhh}.100\%=60\%\)
=> %Fe2O3 =100% - %CuO = 40% .
Vậy ...

a. PTHH: CuO + H2 ---to---> Cu + H2O (1)
Fe2O3 + 3H2 ---to---> 2Fe + 3H2O (2)
Ta có: \(m_{hh}=62,4\left(g\right)\)
=> \(m_{Fe}=62,4-12,8=49,6\left(g\right)\)
b. Theo PT(1): \(n_{H_2}=n_{Cu}=\dfrac{12,8}{64}=0,2\left(mol\right)\)
Theo PT(2):\(n_{H_2}=3.n_{Fe}=3.\dfrac{49,6}{56}\approx2,7\left(mol\right)\)
=> \(n_{H_{2_{\left(2PT\right)}}}=0,2+2,7=2,9\left(mol\right)\)
=> \(V_{H_2}=2,9.22,4=64,96\left(lít\right)\)

a.\(n_{H_2}=\dfrac{6,72}{22,4}=0,3mol\)
\(Fe+2HCl\rightarrow FeCl_2+H_2\)
0,3 0,3 ( mol )
\(m_{Fe}=0,3.56=16,8g\)
\(\%m_{Fe}=\dfrac{16,8}{20}.100=84\%\)
\(\%m_{Cu}=100\%-84\%=16\%\)
b.\(m_{Cu}=20-16,8=3,2g\)
\(n_{Cu}=\dfrac{3,2}{64}=0,05mol\)
\(CuO+H_2\rightarrow\left(t^o\right)Cu+H_2O\)
0,05 0,05 ( mol )
\(m_{CuO}=0,05.80=4g\)
\(n_{H_2}=\dfrac{6,72}{22,4}=0,3\left(mol\right)\\ PTHH:Fe+2HCl\rightarrow FeCl_2+H_2\uparrow\\ Theo.pt:n_{Fe}=n_{H_2}=0,3\left(mol\right)\\ m_{Fe}=0,3.56=16,8\left(g\right)\\ m_{Cu}=20-16,8=3,2\left(g\right)\\ n_{Cu}=\dfrac{3,2}{64}=0,06\left(mol\right)\\ PTHH:CuO+H_2\underrightarrow{t^o}Cu+H_2O\\ Mol:0,05\leftarrow0,05\leftarrow0,05\\ m_{CuO}=0,05.80=4\left(g\right)\)

Chọn C
m o x i t = m K L + m o x i → m o x i = m o x i t – m K L = 24 – 17 , 6 = 6 , 4 g a m .
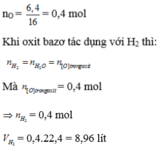

\(n_{H_2}=\dfrac{5,04}{22,4}=0,225\left(mol\right)\)
PTHH: CuO + H2 → Cu + H2O
Mol: x x x
PTHH: Fe2O3 + 3H2 → 2Fe + 3H2O
Mol: y 3y 2y
Ta có hpt:\(\left\{{}\begin{matrix}80x+160y=14\\x+3y=0,225\end{matrix}\right.\Leftrightarrow\left\{{}\begin{matrix}x=0,075\left(mol\right)\\y=0,05\left(mol\right)\end{matrix}\right.\)
\(m_{hh.kim.loại}=m_{Cu}+m_{Fe}=0,075.64+2.0,05.56=10,4\left(g\right)\)
\(n_{H_2}=\dfrac{5,04}{22,4}=0,225\left(mol\right)\)
PTHH:
\(CuO+H_2\underrightarrow{t^o}Cu+H_2O\\ Fe_2O_3+3H_2\underrightarrow{t^o}2Fe+3H_2O\)
Theo 2 pthh trên: \(n_{H_2O}=n_{H_2}=0,225\left(mol\right)\)
\(\rightarrow m_{H_2O}=0,225.18=4,05\left(g\right)\\ \rightarrow m_{H_2}=0,225.2=0,45\left(g\right)\)
Áp dụng ĐLBTKL, ta có:
\(m_{oxit\left(CuO,Fe_2O_3\right)}+m_{H_2}=m_{\text{kim loại}\left(Cu,Fe\right)}+m_{H_2O}\\ \rightarrow m_{\text{kim loại}\left(Cu,Fe\right)}=14+0,45-4,05=10,4\left(g\right)\)

a)
Gọi số mol CuO, Fe2O3 là a, b (mol)
=> 80a + 160b = 40 (1)
\(n_{H_2}=\dfrac{13,44}{22,4}=0,6\left(mol\right)\)
PTHH: CuO + H2 --to--> Cu + H2O
a--->a--------->a
Fe2O3 + 3H2 --to--> 2Fe + 3H2O
b----->3b---------->2b
=> a + 3b = 0,6 (2)
(1)(2) => a = 0,3 (mol);b = 0,1 (mol)
\(\left\{{}\begin{matrix}\%m_{CuO}=\dfrac{0,3.80}{40}.100\%=60\%\\\%m_{Fe_2O_3}=\dfrac{0,1.160}{40}.100\%=40\%\end{matrix}\right.\)
b) nFe = 2b = 0,2 (mol)
PTHH: Fe + 2HCl --> FeCl2 + H2
0,2------------------->0,2
=> VH2 = 0,2.22,4 = 4,48 (l)
\(n_{Pb}=\dfrac{2,07}{207}=0,01mol\)
\(n_{Cu}=\dfrac{1,6}{64}=0,025mol\)
\(PbO+H_2\rightarrow\left(t^o\right)Pb+H_2O\)
0,01 0,01 0,01 ( mol )
\(CuO+H_2\rightarrow\left(t^o\right)Cu+H_2O\)
0,025 0,025 0,025 ( mol )
\(m_{hh}=m_{PbO}+m_{CuO}=\left(0,01.223\right)+\left(0,025.80\right)=4,23g\)
\(V_{H_2}=\left(0,01+0,025\right).22,4=0,784l\)
\(n_{Pb}=\dfrac{2,07}{207}=0,01\left(mol\right)\\ n_{Cu}=\dfrac{1,6}{64}=0,025\left(mol\right)\\ PTHH:PbO+H_2\underrightarrow{t^o}Pb+H_2O\\ Mol:0,01\leftarrow0,01\leftarrow0,01\\ CuO+H_2\underrightarrow{t^o}Cu+H_2O\\ Mol:0,025\leftarrow0,025\leftarrow0,025\)
\(\Rightarrow\left\{{}\begin{matrix}m_{CuO}=0,025.80=2\left(g\right)\\m_{PbO}=0,01.223=2,23\left(g\right)\end{matrix}\right.\Rightarrow m_{oxit}=2+2,23=4,23\left(g\right)\\ V_{H_2}=\left(0,01+0,025\right).22,4=0,784\left(l\right)\)