Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

a) \(m_{Cu}=0,6.64=38,4\left(g\right)\)
b) \(m_{\left(NH4\right)2SO4}=0,8.132=105,6\left(g\right)\)

\(m_{Fe}=0,5.56=28\left(g\right)\)
\(m_{H_2SO_4}=0,75.98=73,5\left(g\right)\)
\(m_{CuSO_4}=0,6.160=96\left(g\right)\)

\(a,n_{\left(NH_4\right)_3PO_4}=0,6\left(mol\right)\\ \Rightarrow n_N=0,6.3=1,8\left(mol\right)\Rightarrow m_N=1,8.14=25,2\left(g\right)\\ n_H=4.3.0,6=7,2\left(mol\right)\Rightarrow m_H=7,2.1=7,2\left(g\right)\\ n_P=n_{hc}=0,6\left(mol\right)\Rightarrow m_P=0,6.31=18,6\left(g\right)\\ n_O=4.0,6=2,4\left(mol\right)\Rightarrow m_O=2,4.16=38,4\left(g\right)\)
\(b,n_S=\dfrac{6,4}{32}=0,2\left(mol\right)\Rightarrow n_{Al_2\left(SO_4\right)_3}=\dfrac{1}{3}.0,2=\dfrac{1}{15}\left(mol\right)\\ \Rightarrow m_{Al_2\left(SO_4\right)_3}=342.\dfrac{1}{15}=22,8\left(g\right)\\ c,n_{Al_2\left(SO_4\right)_3}=\dfrac{20,52}{342}=0,06\left(mol\right)\\ n_O=4.3.0,06=0,72\left(mol\right)\\ \Rightarrow n_{CO_2}=\dfrac{0,72}{2}=0,36\left(mol\right)\Rightarrow V_{CO_2\left(đktc\right)}=0,36.22,4=8,064\left(l\right)\)

a) mN = 0,5 .14 = 7g.
mCl = 0,1 .35.5 = 3.55g
mO = 3.16 = 48g.
b) mN2 = 0,5 .28 = 14g.
mCl2 = 0,1 .71 = 7,1g
mO2 = 3.32 =96g
c) mFe = 0,1 .56 =5,6g mCu = 2,15.64 = 137,6g
mH2SO4 = 0,8.98 = 78,4g.
mCuSO4 = 0,5 .160 = 80g

\(a,m_{Fe_2O_3}=0,15.(56.2+16.3)=0,15.160=24\\ b,m_{MgO}=0,75.(24+16)=0,75.40=30\)

a) \(n_{Fe}=\dfrac{14}{56}=0,25\left(mol\right)\)
\(n_{CaCO_3}=\dfrac{25}{100}=0,25\left(mol\right)\)
\(n_{NaOH}=\dfrac{4}{40}=0,1\left(mol\right)\)
\(n_{...}=\dfrac{1,5.10^{23}}{6.10^{23}}=0,25\left(mol\right)\)
b)
\(m_{ZnSO_4}=0,25.161=40,25\left(g\right)\)
\(m_{AlCl_3}=0,2.133,5=26,7\left(g\right)\)
\(m_{Cu}=0,3.64=19,2\left(g\right)\)
\(m_{Fe_2\left(SO_4\right)_3}=0,35.400=140\left(g\right)\)
d) \(V_{CO_2}=0,2.22,4=4,48\left(l\right)\)
\(V_{Cl_2}=0,15.22,4=3,36\left(l\right)\)
\(V_{SO_2}=0,3.22,4=6,72\left(l\right)\)

a, mCaO = 0,5.56 = 28 (g)
b, \(n_{CO_2}=\dfrac{6,72}{22,4}=0,3\left(mol\right)\)
c, \(n_{H_2SO_4}=\dfrac{24,5}{98}=0,25\left(mol\right)\)
d, \(V_{hhk}=0,2.22,4+0,3.22,4=11,2\left(l\right)\)
e, \(\%m_{Cu}=\dfrac{64}{64+32+16.4}.100\%=40\%\)
Bạn tham khảo nhé!
a) mCaO=nCaO.M(CaO)=0,5.56=28(g)
b) nCO2=V(CO2,dktc)=6,72/22.4=0,3(mol)
c) nH2SO4=mH2SO4/M(H2SO4)=24,5/98=0,25(mol)
d) V(hh H2,NH3)=(0,3+0,2).22,4=11,2(l)
e) %mCu/CuSO4=(64/160).100=40%
Chúc em học tốt!
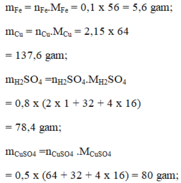
\(a.m_{H_2SO_4}=0,75.98=73,5g\)
\(b.m_{Cu}=0,6.64=38,4g\\ c.m_{\left(NH_4\right)_2SO_4}=0,8.132105,6g\)