Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

a) 2Al + 6HCl --> 2AlCl3 + 3H2
b) \(n_{Al}=\dfrac{8,1}{27}=0,3\left(mol\right)\)
PTHH: 2Al + 6HCl --> 2AlCl3 + 3H2
_____0,3--->0,9---------------->0,45
=> VH2 = 0,45.22,4 = 10,08(l)
c)
\(C\%\left(HCl\right)=\dfrac{0,9.36,5}{100}.100\%=32,85\%\)

nAl=\(\dfrac{5,4}{27}\)=0,2 mol
2Al+3H2SO4→Al2(SO4)3+3H2
0,2-----0,3---------0,1-----------0,3
=>VH2=0,3.22,4=6,72l
=>CMH2SO4=\(\dfrac{0,3}{0,1}\)=3M
=>CM Al2(SO4)3=\(\dfrac{0,1}{0,1}\)=1M

a) \(Pt:2Al+6HCl\rightarrow2AlCl_3+3H_2\)
b) \(n_{Al}=\dfrac{5,4}{27}=0,2mol\)
\(Theopt:n_{H_2}=\dfrac{3}{2}n_{Al}=0,3mol\)
\(\Rightarrow V_{H_2}=0,3.22,4=6,72lít\)
c) \(Theopt:n_{HCl}=3n_{Al}=0,6mol\)
\(\Rightarrow C_Mdd_{HCl}=\dfrac{0,6}{0,2}=3M\)

\(n_{Al}=\dfrac{5,4}{27}=0,2mol\)
\(2Al+3H_2SO_4\rightarrow Al_2\left(SO_4\right)_3+3H_2\)
0,2 0,1 0,3 ( mol )
\(m_{Al_2\left(SO_4\right)_3}=0,1.342=34,2g\)
\(V_{H_2}=0,3.22,4=6,72l\)

\(n_{BaCl_2}=\dfrac{200.20,8\%}{208}=0,2\left(mol\right)\\ PTHH:BaCl_2+H_2SO_4\rightarrow BaSO_4+2HCl\\ n_{BaSO_4}=n_{H_2SO_4}=n_{BaCl_2}=0,2\left(mol\right)\\ a,m_{kt}=m_{BaSO_4}=233.0,2=46,6\left(g\right)\\ b,C\%_{ddH_2SO_4}=\dfrac{0,2.98}{200}.100\%=9,8\%\)

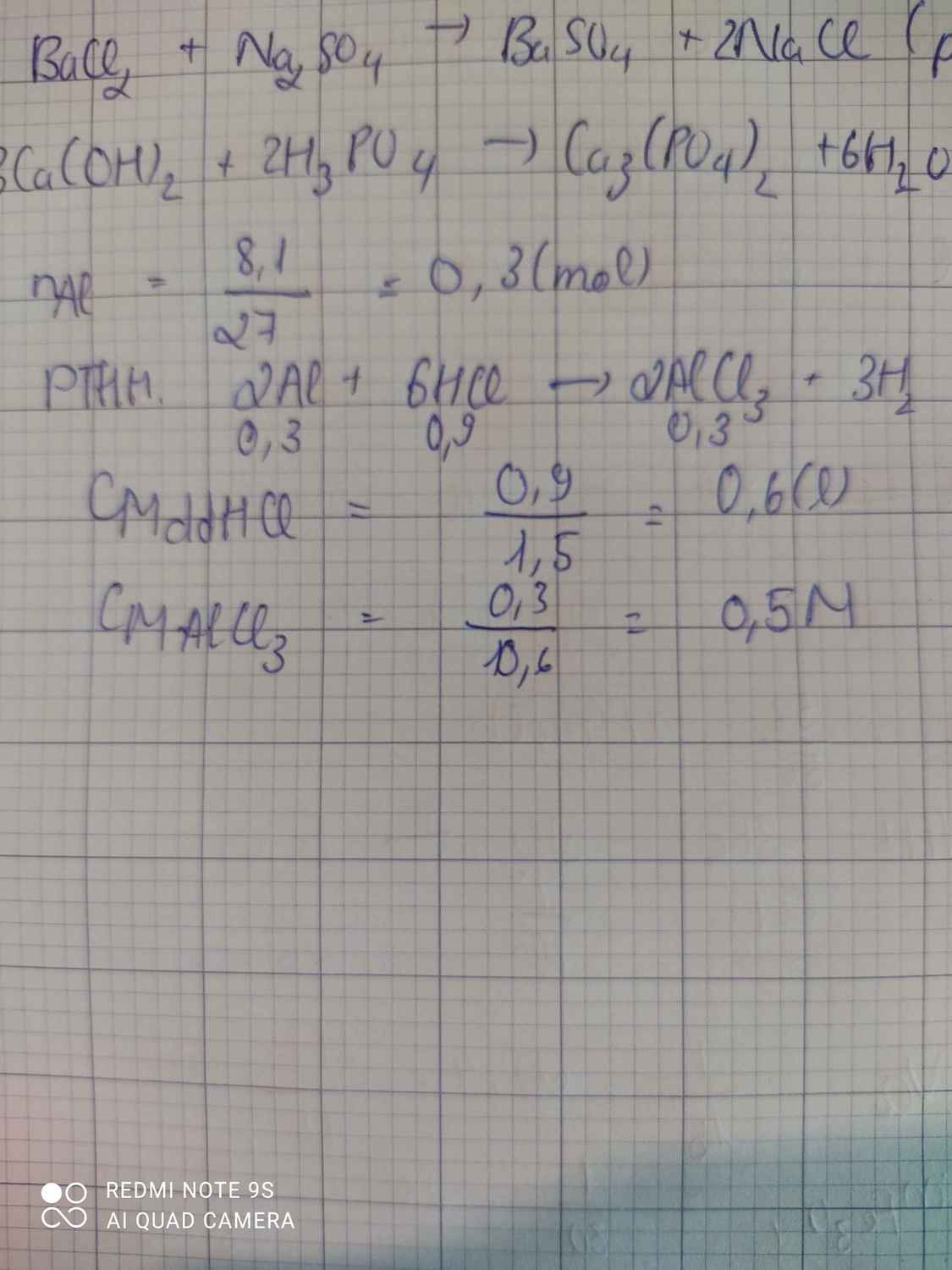
a) \(n_{Al}=\dfrac{8,1}{27}=0,3\left(mol\right)\)
PTHH: 2Al + 6HCl --> 2AlCl3 + 3H2
0,3-->0,9------>0,3--->0,45
=> \(V_{dd.HCl}=\dfrac{0,9}{1,5}=0,6\left(l\right)\)
b) \(C_{M\left(AlCl_3\right)}=\dfrac{0,3}{0,6}=0,5M\)

\(n_{K_2SO_3}=\dfrac{15.8}{158}=0.1\left(mol\right)\)
\(K_2SO_3+H_2SO_4\rightarrow K_2SO_4+SO_2+H_2O\)
\(0.1...........................0.1..........0.1\)
\(V_{SO_2}=0.1\cdot22.4=2.24\left(l\right)\)
\(C_{M_{K_2SO_4}}=\dfrac{0.1}{0.2}=0.5\left(M\right)\)
\(2Al+3H_2SO_4\rightarrow Al_2\left(SO_4\right)_3+3H_2\\ n_{Al}=\dfrac{5,4}{27}=0,2\left(mol\right)\Rightarrow n_{H_2}=\dfrac{3}{2}.0,2=0,3\left(mol\right)\\ V_{H_2\left(đktc\right)}=0,3.22,4=6,72\left(l\right)\)
Câu b thiếu thể tích dd axit nên chưa tính được em