Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

2Fe+ 3Cl2=(t0) 2FeCl3
nFeCl3=16,25/162,5=0,1 mol => nCl2=3/2nFeCl3=3/2.0,1=0,15 mol
2KMnO4+ 16HCl=2KCl+2MnCl2+5Cl2+8H2O
nKMnO4=2/5.nCl2=2/5. 0,15=0,06 mol --> mKMnO4=0.06. 158=9,48 g
nHCl=16/5. nCl2=16/5. 0,15=0,48 mol
--> VddHCl=0,48/ 1=0,48 lit= 480 ml

\(n_{FeCl_3}=\dfrac{16.25}{162.5}=0.1\left(mol\right)\)
\(Fe+\dfrac{3}{2}Cl_2\underrightarrow{t^0}FeCl_3\)
\(......0.15......0.1\)
\(2KMnO_4+16HCl\rightarrow2KCl+2MnCl_2+5Cl_2+8H_2O\)
\(0.06...............0.48........................................0.15\)
\(m_{KMnO_4}=0.06\cdot158=9.48\left(g\right)\)
\(V_{dd_{HCl}}=\dfrac{0.48}{1}=0.48\left(l\right)=480\left(ml\right)\)
\(2Fe+ 3Cl_2 \xrightarrow{t^o} 2FeCl_3\\ n_{Cl_2} = \dfrac{3}{2}n_{FeCl_3} = \dfrac{3}{2}.\dfrac{16,25}{162,5} = 0,15(mol)\\ 2KMnO_4 + 16HCl \to 2KCl + 2MnCl_2 + 5Cl_2 + 8H_2O\\ n_{KMnO_4} = \dfrac{2}{5}n_{Cl_2} = 0,06(mol)\\ \Rightarrow m_{KMnO_4} = 0,06.158 = 9,48(gam)\\ n_{HCl} = \dfrac{16}{4}n_{Cl_2} = 0,48(mol)\\ \Rightarrow V_{dd\ HCl} = \dfrac{0,48}{1} = 0,48(lít) = 480(ml)\)

2KMnO4 + 16HCl ---> 2KCl + 2MnCl2 + 5Cl2 + 8H2O
2Fe + 3Cl2 ---> 2FeCl3
Để điều chế đủ khí Clo thì số mol Cl2 = 3/2 số mol FeCl3 = 1,5.16,25/162,5 = 0,15 mol.
Số mol KMnO4 = 2/5 số mol Cl2 = 0,06 mol; m(KMnO4) = 0,06.158 = 9,48 g
V(HCl) = 0,48/1 = 480 ml.
KMnO4 + 16HCl ---> 2KCl + 2MnCl2 + 5Cl2 + 8H2O
2Fe + 3Cl2 ---> 2FeCl3
Để điều chế đủ khí Clo thì số mol Cl2 = 3/2 số mol FeCl3 = 1,5.16,25/162,5 = 0,15 mol.
Số mol KMnO4 = 2/5 số mol Cl2 = 0,06 mol; m(KMnO4) = 0,06.158 = 9,48 g
V(HCl) = 0,48/1 = 480 ml.

\(n_{Cl_2}=\dfrac{11.2}{22.4}=0.5\left(mol\right)\)
\(2KMnO_4+16HCl_{\left(đ\right)}\rightarrow2KCl+2MnCl_2+5Cl_2+8H_2O\)
\(0.2...................1.6...........................................0.5\)
\(m_{KMnO_4}=0.2\cdot158=31.6\left(g\right)\)
\(V_{dd_{HCl}}=\dfrac{1.6}{1}=1.6\left(l\right)=1600\left(ml\right)\)
\(n_{Cl_2}=\dfrac{11,2}{22,4}=0,5(mol)\\ PTHH:2KMnO_4+16HCl\to 2KCl+2MnCl_2+5Cl_2+8H_2O\\ \Rightarrow n_{KMnO_4}=\dfrac{2}{5}n_{Cl_2}=0,2(mol);n_{HCl}=\dfrac{16}{5}n_{Cl_2}=1,6(mol)\\ \Rightarrow m_{KMnO_4}=0,2.158=31,6(g);V_{dd_{HCl}}=\dfrac{1,6}{1}=1,6(l)=1600(ml)\)

\(2KMnO_4+16HCl\rightarrow2MnCl_2+2KCl+5Cl_2+8H_2O\)
\(2Fe+3Cl_2\rightarrow2FeCl_3\)
Ta có :
\(n_{FeCl3}=\frac{32,5}{56+35,5.3}=0,2\left(mol\right)\)
\(n_{Cl2}=\frac{3}{2}n_{FeCl3}=0,3\left(mol\right)\)
Theo phản ứng:
\(n_{KMnO4}=\frac{2}{5}n_{Cl2}=0,12\left(mol\right)\Rightarrow m_{KMnO4}=0,12.\left(39+55+16.4\right)=96\left(g\right)\)
\(n_{HCl}=\frac{16}{5}n_{Cl2}=0,96\left(mol\right)\)
\(\Rightarrow V_{HCl}=\frac{0,96}{1}=0,96\left(l\right)=960\left(ml\right)\)

\(n_{FeCl_3}=\dfrac{16,25}{162,5}=0,1\left(mol\right)\)
\(2Fe+3Cl_2\rightarrow2FeCl_3\)
0,1 0,15 0,1 (mol)
\(2KMnO_4+16HCl\rightarrow2KCl+2MnCl_2+5Cl_2+8H_2O\)
0,06 0,48 0,15 (mol)
m\(_{KMnO_4}=0,06.158=9,48\left(g\right)\)
\(V_{HCl}=\dfrac{0,48}{1}=0,48\left(l\right)=480\left(ml\right)\)

1. 2Al+3Cl2\(\rightarrow\)2AlCl3
nAlCl3=\(\frac{26,7}{133,5}\)=0,2
\(\rightarrow\)nAl=0,2\(\rightarrow\)mAl=0,2.27=5,4
\(\rightarrow\)nCl2=0,3\(\rightarrow\)VCl2=0,3.22,4=6,72l
2. 2KMnO4+16HCl\(\rightarrow\)2KCl+2MnCl2+5Cl2+8H2O
nFeCl3=\(\frac{16,25}{162,5}\)=0,1
2Fe+3Cl2\(\rightarrow\)2FeCl3
\(\rightarrow\)nCl2=0,1.1,5=0,15
\(\rightarrow\)nKMnO4=\(\frac{0,15.2}{5}\)=0,06
\(\rightarrow\)mKMnO4=0,06.158=9,48
\(\rightarrow\)nHCl=0,48
\(\rightarrow\)VddHCl=0,48l=480ml
Phương trình hóa học của phản ứng:
3Cl2 + 2Fe → 2FeCl3
Theo pt: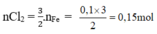
2KMnO4 + 16HCl → 2KCl + 2MnCl2 + 5Cl2 + 8H2O
Theo pt:
mKMnO4 cần = 0,06. 158 = 9,48g