Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

nO (trong CO2) = 2 . nCO2 = 2 . 26,4/44 = 1,2 (mol)
nO (trong H2O) = nH2O = 13,5/18 = 0,75 (mol)
nO (trong O2) = 1,2 + 0,75 = 1,95 (mol)
nO2 = 1,95/2 = 0,975 (mol)
VO2 = 0,975 . 22,4 = 21,84 (l)
Vkk = 21,84 . 5 = 109,2 (l)
nO (trong CO2) = 2 . nCO2 = 2 . 26,4/44 = 1,2 (mol)
nO (trong H2O) = nH2O = 13,5/18 = 0,75 (mol)
nO (trong O2) = 1,2 + 0,75 = 1,95 (mol)
nO2 = 1,95/2 = 0,975 (mol)
VO2 = 0,975 . 22,4 = 21,84 (l)
Vkk = 21,84 . 5 = 109,2 (l)

Gọi x,y lần lượt là số mol CO2 , H2O
\(\left\{{}\begin{matrix}x+y=0,09\\44x+18y=\dfrac{40}{3}.2.0,09\end{matrix}\right.\)
=> \(\left\{{}\begin{matrix}x=0,03\\y=0,06\end{matrix}\right.\)
=> \(n_C=n_{CO_2}=0,03\left(mol\right);n_H=2n_{H_2O}=0,12\left(mol\right)\)
\(BTKL\Rightarrow m_X=0,96\left(g\right)\)
=>\(M_X=\dfrac{0,96}{0,03}=32\)
\(BTNT\left(O\right):n_{O\left(trongX\right)}=0,03.2+0,06-0,045.2=0,03\left(mol\right)\)
Gọi CT của X : CxHyOz
x : y : z =0,03 : 0,12 : 0,03 = 1:4:1
=> CTĐGN : (CH4O)n
Mà \(M_X=32n=32\)
=> n=1
=> CT của X : CH4O

\(n_C=\dfrac{1,2}{12}=0,1\left(mol\right)\)
PTHH: C + O2 --to--> CO2
a-->a--------->a
2C + O2 --to--> 2CO
b--->0,5b------>b
=> a + b = 0,1
Có: \(\overline{M}_X=\dfrac{44a+28b}{a+b}=16.2=32\)
=> a = 0,025; b = 0,075
\(n_{O_2}=a+0,5b=0,0625\left(mol\right)\)
=> \(V_{O_2}=0,0625.22,4=1,4\left(l\right)\)

\(n_{H_2O}=\dfrac{4,48}{22,4}=0,2\left(mol\right)\)
\(2H_2+O_2\underrightarrow{t^o}2H_2O\)
0,2 0,2
=> \(V_{H_2}=0,2.22,4=4,48\left(l\right)\\
\%V_{H_2}=\dfrac{4,48}{4,48+6,72}.100\%=40\%\\
\Rightarrow\%V_{O_2}=100\%-40\%=60\%\)

\(n_{O_2}=\dfrac{11,2}{22,4}=0,5\left(mol\right)\)
\(n_{CO_2}=\dfrac{6,72}{22,4}=0,3\left(mol\right)\)
\(CO+\dfrac{1}{2}O_2\underrightarrow{t^o}CO_2\)
0,3 0,3
\(n_{H_2}=0,5-0,3=0,2\left(mol\right)\)
\(\%_{V_{CO}}=\dfrac{0,3.22,4.100}{11,2}=60\%\)
\(\%_{V_{H_2}}=\dfrac{0,2.22,4.100}{11,2}=40\%\)
☕T.Lam

CxHy:a(mol)
CO:b(mol)
=>a+b\(=\dfrac{6,72}{22,4}\)=0,3(mol)
nCO2=\(\dfrac{22}{44}\)=0,5(mol)
nH2O=\(\dfrac{7,2}{18}\)=0,4(mol)
nO2=\(\dfrac{13,44}{22,4}\)=0,6(mol)
Bảo toàn C: ax + b = 0,5
Bảo toàn H: ay = 0,8
Bảo toàn O: b + 0,6.2 = 0,5.2 + 0,4
=> b = 0,2 (mol)
=> a = 0,1 (mol)
=> x = 3 ; y = 8 => CTPT: C3H8
%VC3H8=\(\dfrac{0,1}{0,3}\).100%=33,33%
%VCO=\(\dfrac{0,2}{0,3}\).100%=66,67%

\(2CO + O_2 \xrightarrow{t^o} 2CO_2(1)\\ 2H_2 + O_2 \xrightarrow{t^o} 2H_2O(2)\\ n_{CO} = n_{CO_2} = \dfrac{4,48}{22,4} = 0,2(mol)\\ n_{O_2(1)} = \dfrac{n_{CO_2}}{2} = 0,1(mol)\\ \Rightarrow n_{H_2} = 2n_{O_2(2)} = 2.(\dfrac{6,72}{22,4}-0,1)=0,4(mol)\\ \%V_{CO} = \dfrac{0,2}{0,2+0,4}.100\% = 33,33\%\\ \%V_{H_2} = 100\% -33,33\% = 66,67\%\)
\(\%m_{CO} = \dfrac{0,2.28}{0,2.28+0,4.2}.100\% = 87,5\%\\ \%m_{H_2} = 100\% - 87,5\% = 12,5\%\)
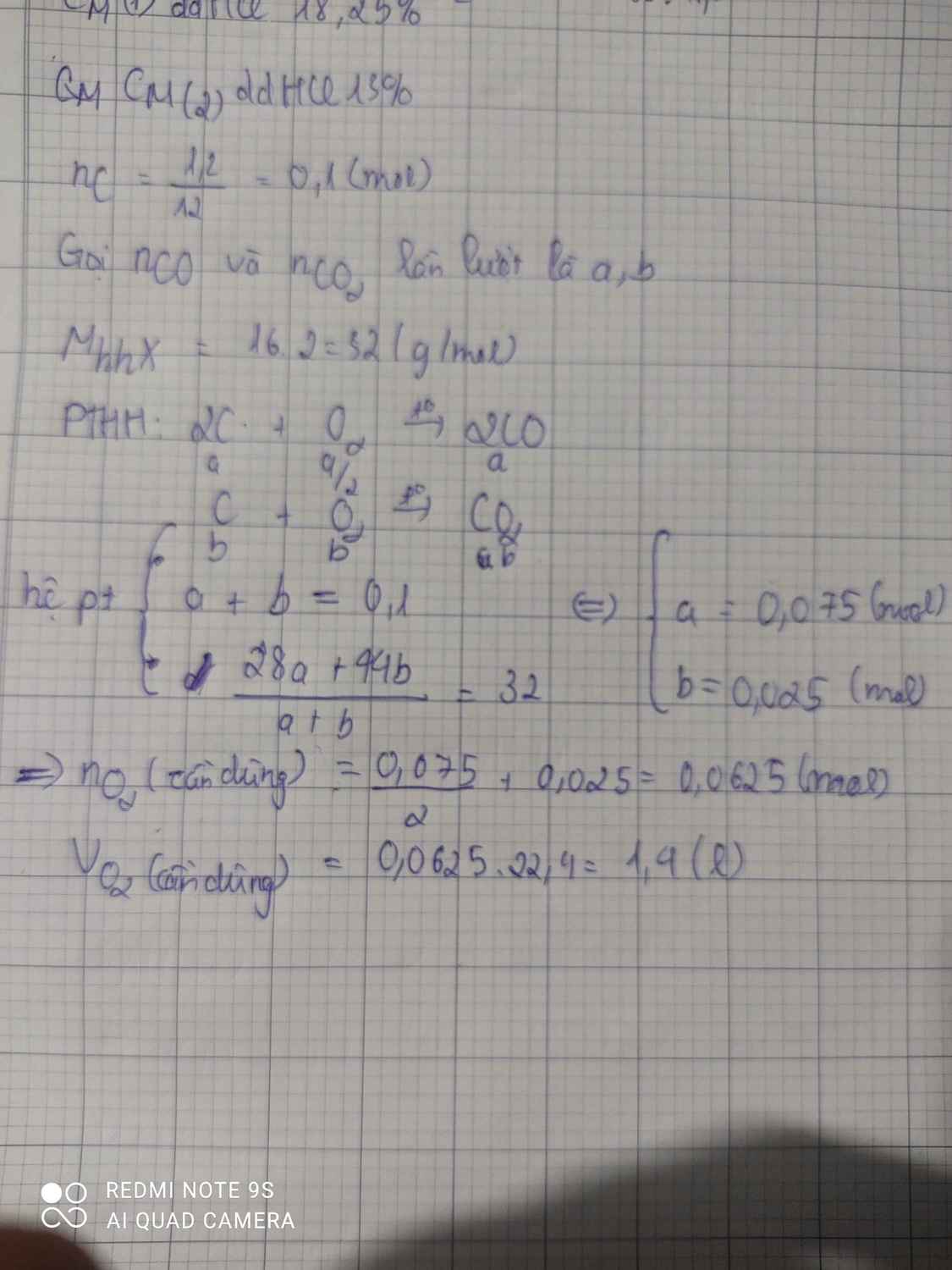
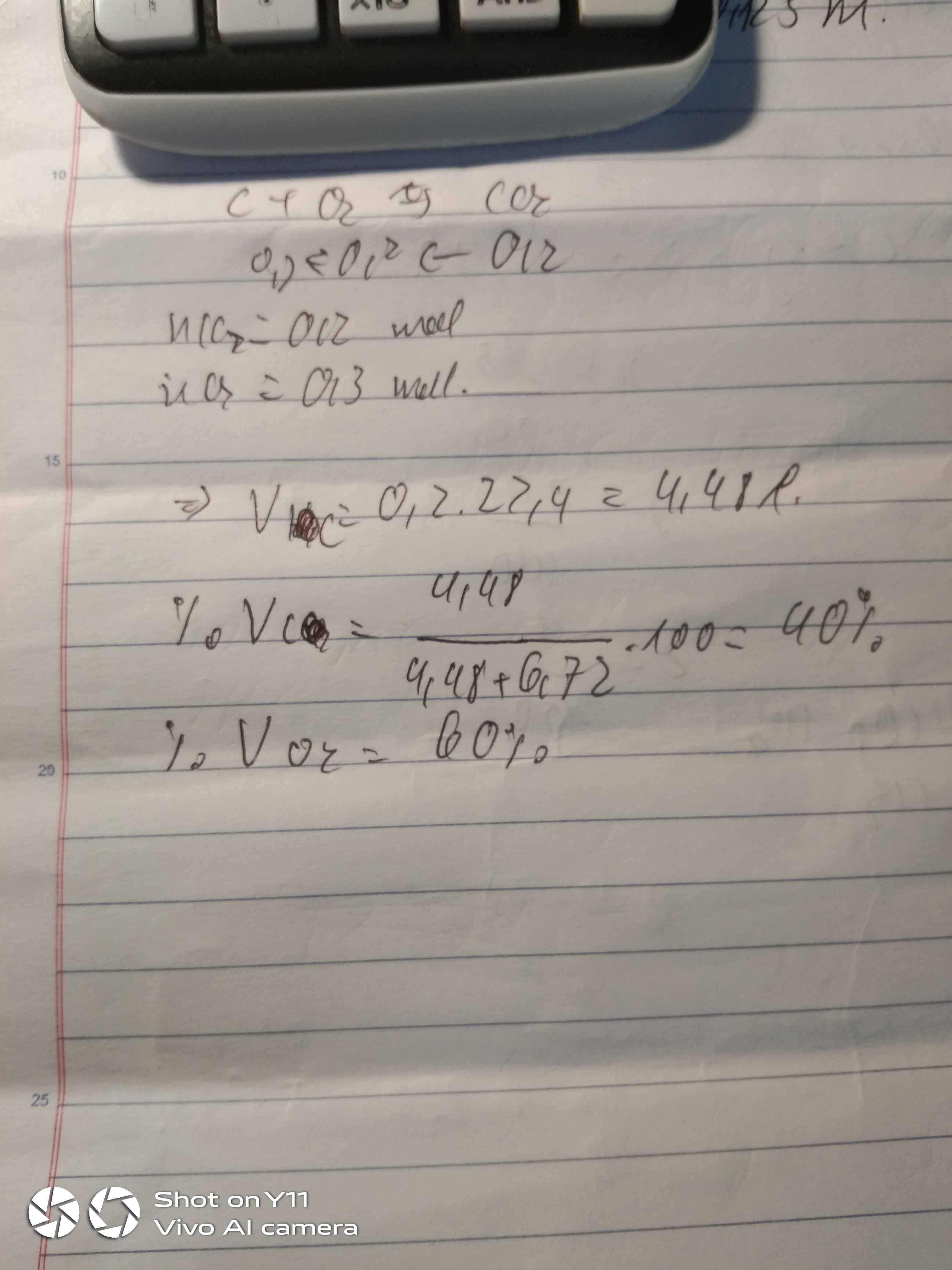
\(CO+\dfrac{1}{2}O_2\underrightarrow{to}CO_2\\ H_2+\dfrac{1}{2}O_2\underrightarrow{to}H_2O\\ n_{O_2}=\dfrac{1}{2}.\left(n_{CO}+n_{H_3}\right)=\dfrac{0,3}{2}=0,15\left(mol\right)\\ V=V_{O_2\left(đktc\right)}=0,15.22,4=3,36\left(l\right)\)