Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

pứ: Fe + 2HCl -> FeCl2 + H2
b. nFe = \(\dfrac{5,6}{56}\)= 0,1 mol
Từ pt suy ra được: nHCl = 2.nFe= 0,2 mol
=> mHCl = 0,2. 36,5 = 7,3 g
c. nH2 = nFe = 0,1 mol
=> VH2 = 0,1.22,4 = 2,24 (lít)

\(nAl=\dfrac{2,7}{27}=0,1\left(mol\right)\)
\(mHCl=\dfrac{200.7,3\%}{100\%}=14,6\left(g\right)\)
\(nHCl=\dfrac{14,6}{36,5}=0,4\left(mol\right)\)
PTHH:
\(2Al+6HCl\rightarrow2AlCl_2+3H_2\)
2 6 2 3 (mol)
0,1 0,3 0,1 0,15 (mol)
LTL : 0,1 / 2 < 0,4/6
=> Al đủ , HCl dư
1. \(VH_2=0,15.22,4=3,36\left(l\right)\)
2. \(mH_2=0,15.2=0,3\left(g\right)\)
mdd = mAl + mddHCl - mH2 = 2,7 + 200 - 0,3 = 202,4 (g)
\(mH_2SO_{4\left(dưsaupứ\right)}=0,1.98=9,8\left(g\right)\)
\(mAlCl_2=0,1.98=9,8\left(g\right)\)
\(C\%_{ddH_2SO_4}=\dfrac{9,8.100}{202,4}=4,84\%\)
\(C\%_{AlCl_2}=\dfrac{9,8.100}{202,4}=4,84\%\)

\(n_{Fe}=\dfrac{1,68}{56}=0,03\left(mol\right)\\ Fe+2HCl\rightarrow FeCl_2+H_2\\ 0,03....0,06.....0,03.......0,03\left(mol\right)\\ a,V_{H_2\left(đktc\right)}=0,03.22,4=0,672\left(l\right)\\ b,m_{HCl}=0,06.36,5=2,19\left(g\right)\\ c,m_{FeCl_2}=127.0,03=3,81\left(g\right)\)

a) \(n_{Fe}=\dfrac{12}{56}=\dfrac{3}{14}\left(mol\right)\)
PTHH: \(Fe+2HCl\rightarrow FeCl_2+H_2\)
\(\dfrac{3}{14}\)---------------------->\(\dfrac{3}{14}\)
\(\Rightarrow V_{H_2}=\dfrac{3}{14}.22,4=4,8\left(l\right)\)
b) \(n_{ZnO}=\dfrac{8,1}{81}=0,1\left(mol\right)\)
PTHH: \(ZnO+H_2\xrightarrow[]{t^o}Zn+H_2O\)
Xét tỉ lệ: \(0,1< \dfrac{3}{14}\Rightarrow H_2\) dư
Theo PT: \(n_{Zn}=n_{ZnO}=0,1\left(mol\right)\Rightarrow m_{Zn}=0,1.65=6,5\left(g\right)\)
\(Fe+2HCl\rightarrow FeCl_2+H_2\)
\(1mol\) \(1mol\)
\(\dfrac{3}{14}mol\) \(\dfrac{3}{14}mol\)
\(a)n_{Fe}=\dfrac{m}{M}=\dfrac{12}{56}\approx0,21=\dfrac{3}{14}\left(mol\right)\)
\(V_{H_2}=n.22,4=\dfrac{3}{14}.22,4=4,8\left(l\right)\)
\(b)n_{ZnO}=\dfrac{m}{M}=\dfrac{8,1}{81}=0,1\left(mol\right)\)
\(ZnO+H_2\rightarrow Zn+H_2O\)
\(1mol\) \(1mol\) \(1mol\)
\(0,1mol\) \(0,1mol\) \(0,1mol\)
\(\text{Ta thấy }H_2\text{ dư,ZnO phản ứng hết.Bài toán tính theo ZnO}\)
\(m_{Zn}=n.M=0,1.65=6,5\left(g\right)\)

\(n_{Zn}=\dfrac{m}{M}=\dfrac{3,25}{65}=0,05\left(mol\right)\)
\(PTHH:Zn+2HCl\rightarrow ZnCl_2+H_2\)
1 2 1 1
0,05 0,1 0,05 0,05
a) \(V_{H_2}=n.24,79=0,05.24,79=1,2395\left(l\right)\)
\(m_{ZnCl_2}=n.M=0,05.\left(65+35,5.2\right)=6,8\left(g\right)\)
b) \(PTHH:CuO+H_2\underrightarrow{t^o}Cu+H_2O\)
Ta cos tỉ lệ: \(\dfrac{0,1}{1}>\dfrac{0,05}{1}\Rightarrow\) CuO dư.
Theo ptr, ta có: \(n_{Cu}=n_{H_2}=0,05mol\\ \Rightarrow m_{Cu}=n.M=0,05.64=3,2\left(g\right).\)
a, PT: \(Zn+2HCl\rightarrow ZnCl_2+H_2\)
Ta có: \(n_{Zn}=\dfrac{3,25}{65}=0,05\left(mol\right)\)
THeo PT: \(n_{ZnCl_2}=n_{H_2}=n_{Zn}=0,05\left(mol\right)\)
\(\Rightarrow V_{H_2}=0,05.24,79=1,2395\left(l\right)\)
\(m_{ZnCl_2}=0,05.136=6,8\left(g\right)\)
b, Ta có: \(n_{CuO}=\dfrac{8}{80}=0,1\left(mol\right)\)
PT: \(CuO+H_2\underrightarrow{t^o}Cu+H_2O\)
Xét tỉ lệ: \(\dfrac{0,1}{1}>\dfrac{0,05}{1}\), ta được CuO dư.
Theo PT: \(n_{Cu}=n_{H_2}=0,05\left(mol\right)\Rightarrow m_{Cu}=0,05.64=3,2\left(g\right)\)
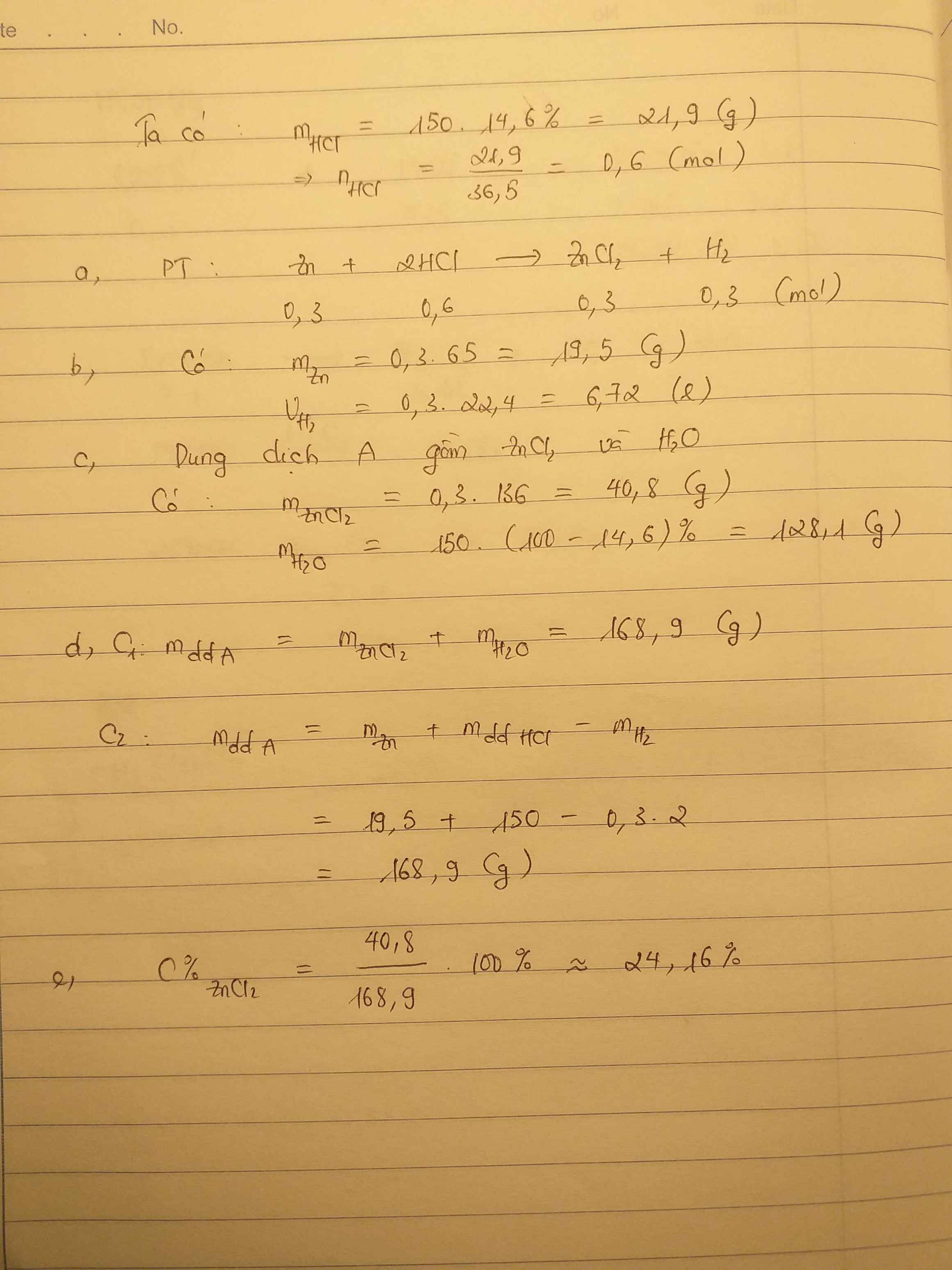

nH2 = \(\dfrac{11,1555}{24,79}=0,45\left(mol\right)\)
PTHH: 2M + 2xHCl -> 2MClx + xH2
0,9 <-------------- 0,45
mHCl = 0,9 . 36,5 = 32,85 (g)
mH2 = 0,45 . 2 = 0,9 (g)
Áp dụng ĐLBTKL, ta có:
mM + mHCl = mMClx + mH2
=> mM = 40,04 + 0,9 - 32,85 = 8,09 (g)
%mM = \(\dfrac{8,09}{10}=80,9\%\)