Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

\(n_{Mg}=\dfrac{6}{24}=0,25\left(mol\right)\\ Mg+2HCl\rightarrow MgCl_2+H_2\\ 0,25.........0,5.........0,25.......0,25\left(mol\right)\\ a.V_{H_2\left(đktc\right)}=0,25.22,4=5,6\left(l\right)\\ b.m_{HCl}=0,5.36,5=18,25\left(g\right)\\ c.n_{Fe_2O_3}=\dfrac{16}{160}=0,1\left(mol\right)\\ Fe_2O_3+3H_2\underrightarrow{^{to}}2Fe+3H_2O\\ Vì:\dfrac{0,25}{3}< \dfrac{0,1}{1}\\ \Rightarrow Fe_2O_3dư\\ n_{Fe}=\dfrac{2}{3}.0,25=\dfrac{1}{6}\left(mol\right)\\ \Rightarrow m_{Fe}=\dfrac{1}{6}.56\approx9,333\left(g\right)\)
a,\(n_{Mg}=\dfrac{6}{24}=0,25\left(mol\right)\)
PTHH: Mg + 2HCl → MgCl2 + H2
Mol: 0,25 0,5 0,25
\(\Rightarrow V_{H_2}=0,25.22,4=5,6\left(l\right)\)
b,\(m_{HCl}=0,5.36,5=18,25\left(g\right)\)
c,\(n_{Fe_2O_3}=\dfrac{16}{160}=0,1\left(mol\right)\)
PTHH: Fe2O3 + 3H2 → 2Fe + 3H2O
Mol: 0,25 \(\dfrac{1}{6}\)
Ta có: \(\dfrac{0,1}{1}>\dfrac{0,25}{3}\)⇒ Fe2O3 dư, H2 hết
\(m_{Fe}=\dfrac{1}{6}.56=9,33\left(g\right)\)

a) nAl=2,7/27=0,1(mol)
nHCl=14,6/36,5= 0,4(mol)
PTHH: 2Al +6 HCl -> 2 AlCl3 +3 H2
Ta có: 0,1/2 < 0,6/4
=> HCl dư, Al hết, tính theo nAl
=> nAlCl3=nAl=0,1(mol)
=> mAlCl3=0,1.133,5=13,35(g)
b) nH2= 3/2. nAl=3/2. 0,1=0,15(mol)
=>V(H2,đktc)=0,15.22,4=3,36(l)
c) mFe2O3(nguyên chất)= 80%. 38,4=30,72(g)
=>nFe2O3= 30,72/160=0,192(mol)
PTHH: Fe2O3 + 3 H2 -to->2 Fe +3 H2O
Ta có: 0,192/1 > 0,15/3
=> H2 hết, Fe2O3 dư, tính theo nH2
=> nFe= 2/3. nH2= 2/3. 0,15=0,1(mol)
=>mFe=0,1.56=5,6(g)
a,\(n_{Al}=\dfrac{2,7}{27}=0,1\left(mol\right);n_{HCl}=\dfrac{14,6}{36,5}=0,4\left(mol\right)\)
PTHH: 2Al + 6HCl → 2AlCl3 + 3H2
Mol: 0,1 0,1 0,15
Tỉ lệ:\(\dfrac{0,1}{2}< \dfrac{0,4}{6}\) ⇒ Al pứ hết,HCl dư
\(\Rightarrow m_{AlCl_3}=0,1.133,5=13,35\left(g\right)\)
b,\(V_{H_2}=0,15.22,4=3,36\left(l\right)\)
c,\(m_{Fe_2O_3\left(tinhkhiét\right)}=38,4.\left(100\%-20\%\right)=30,72\left(g\right)\)
⇒\(n_{Fe_2O_3}=\dfrac{30,72}{160}=0,192\left(mol\right)\)
PTHH: Fe2O3 + 3H2 → 2Fe + 3H2O
Mol : 0,15 0,1
Tỉ lệ:\(\dfrac{0,192}{1}>\dfrac{0,15}{3}\)⇒ Fe2O3 dư,H2 hết
=> mFe = 0,1.56 =5,6 (g)

a) Zn + 2HCl → ZnCl2 + H2
nZn = 9,75 : 65 = 0,15 mol
Theo ptpư
nH2 = nZn = 0,15 mol
VH2 = 0,15 . 22,4 = 3,36 lit
b) CuO + H2 →H2O + Cu
nCuO = 20 : 80 = 0,25 mol
nCuO p/ư = nH2 = 0,15 mol
=> Dư CuO
nCu thu được= nH2 = 0,15 mol
mCu= 0,15 x 64 = 9,6 gam

Dung dịch Ca(OH)2 không hấp thụ khí CO nên 6,72 lít khí thoát ra chính là khí CO dư.

nCH3COOH=48/60=0,8 mol
2CH3COOH + Fe --> (CH3COO)2Fe + H2
0,8 0,4 0,4 mol
=>m(CH3COO)2Fe=0,4*174=69,6 g
2H2 +O2 --> 2H2O
0,4 0,2 mol
=>VO2=0,2*22,4=4,48 lít
=> V không khí =4,48*5=22,4 lít
PT: \(2CH_3COOH+Fe\rightarrow\left(CH_3COO\right)_2Fe+H_2\)
a, Ta có: \(n_{CH_3COOH}=\dfrac{4,8}{60}=0,08\left(mol\right)\)
Theo PT: \(n_{\left(CH_3COO\right)_2Fe}=\dfrac{1}{2}n_{CH_3COOH}=0,04\left(mol\right)\)
\(\Rightarrow m_{\left(CH_3COO\right)_2Fe}=0,04.174=6,96\left(g\right)\)
b, Theo PT: \(n_{H_2}=\dfrac{1}{2}n_{CH_3COOH}=0,04\left(mol\right)\)
PT: \(2H_2+O_2\underrightarrow{t^o}2H_2O\)
___0,04__0,02 (mol)
\(\Rightarrow V_{O_2}=0,02.22,4=0,448\left(l\right)\)
\(\Rightarrow V_{kk}=0,448.5=2,24\left(l\right)\)
Bạn tham khảo nhé!

\(a,PTHH:Fe+2HCl\rightarrow FeCl_2+H_2\\ n_{Fe}=\dfrac{22,4}{56}=0,4\left(mol\right)\\ b,n_{FeCl_2}=n_{H_2}=n_{Fe}=0,4\left(mol\right)\\ V_{H_2\left(đktc\right)}=0,4.22,4=8,96\left(l\right)\\ c,m_{FeCl_2}=127.0,4=50,8\left(g\right)\)

nZn= 19,5/65=0,3(mol); nFe2O3=19,2/160=0,12(mol)
PTHH: Zn + 2 HCl -> ZnCl2 + H2
Fe2O3 + 3 H2 -to-> 2 Fe +3 H2O
nH2=nZnCl2= nZn=0,3(mol) => V(H2,đktc)=0,3.22,4= 6,72(l)
b) nHCl= 2.0,3=0,6(mol) => mHCl=0,6.36,5=21,9(g)
=>mddHCl=(21,9.100)/20=109,5(g)
=>m=109,5(g)
c) mH2=0,3.2=0,6(mol)
mddZnCl2=19,5+109,5 - 0,6= 128,4(g)
mZnCl2=0,3. 136= 40,8(g)
=>C%ddZnCl2= (40,8/128,4).100=31,776%
d) Ta có: 0,3/3 < 0,12/1
=> H2 hết, Fe2O3 dư, tính theo nH2
=> nFe= 2/3. nH2= 2/3. 0,3= 0,2(mol)
=>mFe=0,2.56=11,2(g)
a, nZn = 19,5/65=0,3 (mol)
PTHH: Zn + 2HCl → ZnCl2 + H2
Mol: 0,3 0,15 0,3 0,3
=> \(V_{H_2}=0,3.22,4=6,72\left(l\right)\)
b,mHCl=0,15.36,5=5,475 (g)
=> m=mddHCl=5,475:20%=27,375 (g)
c,mdd sau pứ =19,5+27,375=46,875 (g)
\(m_{ZnCl_2}=0,3.136=40,8\left(g\right)\)
\(\Rightarrow C\%_{ZnCl_2}=\dfrac{40,8}{46,875}.100\%=87,04\%\)
d,\(n_{Fe_2O_3}=\dfrac{19,2}{160}=0,12\left(mol\right)\)
PTHH: Fe2O3 + 3H2 → 2Fe + 3H2O
Mol: 0,3 0,2
Tỉ lệ: 0,12/1>0,3/3 ⇒ Fe2O3 dư,H2 pứ hết
=> mFe=0,2.56=11,2 (g)

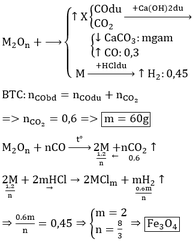
$CO + O_{oxit} \to CO_2$
$CO_2 + Ca(OH)_2 \to CaCO_3 + H_2O$
$n_{O(oxit)} = n_{CaCO_3} = \dfrac{8}{100} = 0,08(mol)$
$Fe + H_2SO_4 \to FeSO_4 + H_2$
$n_{Fe} = n_{H_2} = \dfrac{1,344}{22,4} = 0,06(mol)$
Ta có :
$n_{Fe} : n_O = 0,06 : 0,08 = 3 : 4$
Vậy oxit là $Fe_3O_4$
Công thức oxit sắt có dạng: \(Fe_xO_y\)
\(Fe_xO_y+yCO\rightarrow xFe+yCO_2\uparrow\)
\(Fe+H_2SO_4\rightarrow FeSO_4+H_2\uparrow\)
\(\Rightarrow n_{Fe}=n_{H_2}=\dfrac{1,344}{22,4}=0,06\left(mol\right)\)
\(CO_2+Ca\left(OH\right)_2\rightarrow CaCO_3\downarrow+H_2O\)
\(\Rightarrow n_{CO}=n_{CO_2}=n_{CaCO_3}=0,08\left(mol\right)\)
\(\Rightarrow n_{O\left(Fe_xO_y\right)}=n_{O\left(CO_2\right)}-n_{O\left(CO\right)}=2n_{CO_2}-n_{CO}=0,08\left(mol\right)\)
\(\Rightarrow n_{Fe}:n_O=0,06:0,08=3:4\)
\(\Rightarrow Fe_3O_4\)

a) \(n_{HCl}=0,4.1=0,4\left(mol\right)\)
PTHH: Fe + 2HCl → FeCl2 + H2
Mol: 0,2 0,4 0,2
b, \(V_{H_2}=0,2.22,4=4,48\left(l\right)\)
\(m_{Fe}=0,2.56=11,2\left(g\right)\)
c, \(n_{Cu\left(tt\right)}=\dfrac{10,24}{64}=0,16\left(mol\right)\)
PTHH: H2 + CuO → Cu + H2O
Mol: 0,2 0,2
\(\Rightarrow H=\dfrac{n_{Cu\left(tt\right)}}{n_{Cu\left(lt\right)}}=\dfrac{0,16}{0,2}.100\%=80\%\)
a)
$2Al + 6HCl \to 2AlCl_3 + 3H_2$
$n_{Al} = \dfrac{10,8}{27} = 0,4(mol)$
Theo PTHH : $n_{H_2} = \dfrac{3}{2}n_{Al} = 0,6(mol)$
$V_{H_2} = 0,6.22,4 = 13,44(lít)$
b)
$Fe_2O_3 + 3H_2 \xrightarrow{t^o} 2Fe + 3H_2O$
Theo PTHH : $n_{Fe} = \dfrac{2}{3}n_{H_2} = 0,4(mol)$
$m_{Fe} = 0,4.56 = 22,4(gam)$