Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

\(n_{H_2}=\dfrac{17.92}{22.4}=0.8\left(mol\right)\)
\(n_{Fe_3O_4}=\dfrac{69.6}{232}=0.3\left(mol\right)\)
\(Fe_3O_4+4H_2\underrightarrow{t^0}3Fe+4H_2O\)
\(0.2..............0.8\)
\(m_{Fe_3O_4\left(dư\right)}=\left(0.3-0.2\right)\cdot232=23.2\left(g\right)\)
\(Zn+2HCl\rightarrow ZnCl_2+H_2\)
\(0.8......................................0.8\)
\(m_{Zn}=0.8\cdot65=52\left(g\right)\)

\(n_P=\dfrac{m}{M}=0,2\left(mol\right)\)
- Ta có : \(V_{O_2}=\dfrac{V_{kk}}{5}=4,48\left(l\right)\)
\(n_{O_2}=\dfrac{V}{22,4}=0,2\left(mol\right)\)
\(4P+5O_2\rightarrow2P_2O_5\)
- Theo phương pháp đường chéo ta có :
=> Sau phản ứng O2 phản ứng hết, P còn dư ( dư 0,04 mol )
Vậy sau phản ứng photpho không cháy hết .
b, - Chất được tạo thành là P2O5 .
Theo PTHH : \(n_{P2O5}=\dfrac{n_P}{2}=\dfrac{0,16}{2}=0,08\left(mol\right)\)
\(\Rightarrow m_{P2O5}=n.M=11,36\left(g\right)\)

\(n_{Fe}=\dfrac{11,2}{56}=0,2\left(mol\right)\) ; \(n_{O_2}=\dfrac{5,6}{22,4}=0,25\left(mol\right)\)
\(3Fe+2O_2\rightarrow\left(t^o\right)Fe_3O_4\)
0,2 < 0,25 ( mol )
0,2 \(\dfrac{2}{15}\) \(\dfrac{1}{15}\) ( mol )
`->` Chất dư là O2
\(m_{O_2\left(dư\right)}=\left(0,25-\dfrac{2}{15}\right).32=3,73\left(g\right)\)
\(V_{kk}=V_{O_2}.5=\dfrac{2}{15}.22,4.5=14,93\left(l\right)\)
\(m_{bôt.sắt}=\dfrac{11,2.100}{100-12}=12,72\left(g\right)\)

nP= 6,2 : 31 = 0,2 (MOL)
nO2 = 6,4 : 32= 0,2 (mol)
pthh : 4P+5O2 -t--> 2P2O5
LTL
0,2/4 > 0,2/5
=> P du
2
nO2 = 2,24 : 22,4 =0,1 (mol)
pthh : KMnO4 -t-> K2MnO4 + MnO2 + O2
0,2 <-------------------------------0,1 (mol)
mKMnO4 = 0,2 . 158 = 31,6 (g)

\(n_P=\dfrac{3,1}{31}=0,1\left(mol\right)\)
\(n_{O_2}=\dfrac{4,48}{22,4}=0,2mol\)
4P + 5O2 \(\underrightarrow{t^o}\) 2P2O5
\(\dfrac{0,1}{4}< \dfrac{0,2}{5}\) => O2 dư, Photpho đủ
\(n_{O_2}=0,2-0,04=0,16\left(mol\right)\)
\(m_{P_2O_5}=\) 0,05 . 142 = 7,1 ( g )

nP=\(\dfrac{62}{31}\)=0,2(mol)
nO2=\(\dfrac{7,84}{22,4}\)=0,35(mol)
PTHH:4P+5O2to→2P2O5
tpứ: 0,2 0,35
pứ: 0,2 0,25 0,1
spứ: 0 0,1 0,1
a)chất còn dư là oxi
mO2dư=0,1.32=3,2(g)
b)mP2O5=n.M=0,1.142=14,2(g)
\(a.n_P=0,2\left(mol\right);n_{O_2}=0,35\left(mol\right)\\ 4P+5O_2-^{t^o}\rightarrow2P_2O_5\\ LTL:\dfrac{0,2}{4}< \dfrac{0,35}{5}\\ \Rightarrow SauphảnứngO_2dư\\ n_{O_2\left(pứ\right)}=\dfrac{5}{4}n_P=0,25\left(mol\right)\\ \Rightarrow m_{P\left(dư\right)}=\left(0,35-0,25\right).32=3,2\left(g\right)\\ b.n_{P_2O_5}=\dfrac{1}{2}n_P=0,1\left(mol\right)\\ \Rightarrow m_{P_2O_5}=0,1.142=14,2\left(g\right)\)

a) \(n_P=\dfrac{18,6}{31}=0,6\left(mol\right)\)
\(n_{O_2}=\dfrac{33,6}{22,4}.\dfrac{1}{5}=0,3\left(mol\right)\)
PTHH: 4P + 5O2 --to--> 2P2O5
Xét tỉ lệ: \(\dfrac{0,6}{4}>\dfrac{0,3}{5}\) => P dư, O2 hết
PTHH: 4P + 5O2 --to--> 2P2O5
0,24<-0,3-------->0,12
=> mP(dư) = (0,6 - 0,24).31 = 11,16 (g)
b) mP2O5 = 0,12.142 = 17,04 (g)
nP = 18,6 : 0,6 (mol)
nO2 = (33,6 : 22,4 ) . 21% = 0,315 (mol)
pthh : 4P +5O2 -t-> 2P2O5
LTL :
0,6 / 4 < 0,315 / 5
=> P dư
nP (pư) = nO2 = 0,315 (mol)
nP(d) = nP(bđ) - nP (pư) = 0,6 - 0,315 = 0,285 (mol)
=> mP (dư ) = 0,285 . 31 = 8,935 (g)
theo pthh nP2O5 = 2/5 nO2 = 0,126 ( mol)
=> mP2O5 = 0,126 . 142 = 17,892 (g)

a. \(n_P=\dfrac{12.4}{31}=0,4\left(mol\right)\)
\(n_{O_2}=\dfrac{67.2}{22,4}=3\left(mol\right)\)
Ta thấy : 0,4 < 3 => P đủ , O2 dư
PTHH : 4P + 5O2 -----to-----> 2P2O5
0,4 0,5 0,2
b. \(m_{P_2O_5}=0,2.142=28,4\left(g\right)\)
sai một chỗ là ta thấy \(\dfrac{0,4}{4}< \dfrac{3}{5}\) nha bạn!!

\(1,2H_2+O_2\underrightarrow{t}2H_2O\)
\(2Mg+O_2\underrightarrow{t}2MgO\)
\(2Cu+O_2\underrightarrow{t}2CuO\)
\(S+O_2\underrightarrow{t}SO_2\)
\(4Al+3O_2\underrightarrow{t}2Al_2O_3\)
\(C+O_2\underrightarrow{t}CO_2\)
\(4P+5O_2\underrightarrow{t}2P_2O_5\)
\(2,PTHH:C+O_2\underrightarrow{t}CO_2\)
\(a,n_{O_2}=0,2\left(mol\right)\Rightarrow n_{CO_2}=0,2\left(mol\right)\Rightarrow m_{CO_2}=8,8\left(g\right)\)
\(b,n_C=0,3\left(mol\right)\Rightarrow n_{CO_2}=0,3\left(mol\right)\Rightarrow m_{CO_2}=13,2\left(g\right)\)
c, Vì\(\frac{0,3}{1}>\frac{0,2}{1}\)nên C phản ửng dư, O2 phản ứng hết, Bài toán tính theo O2
\(n_{O_2}=0,2\left(mol\right)\Rightarrow n_{CO_2}=0,2\left(mol\right)\Rightarrow m_{CO_2}=8,8\left(g\right)\)
\(3,PTHH:CH_4+2O_2\underrightarrow{t}CO_2+2H_2O\)
\(C_2H_2+\frac{5}{2}O_2\underrightarrow{t}2CO_2+H_2O\)
\(C_2H_6O+3O_2\underrightarrow{t}2CO_2+3H_2O\)
\(4,a,PTHH:4P+5O_2\underrightarrow{t}2P_2O_5\)
\(n_P=1,5\left(mol\right)\Rightarrow n_{O_2}=1,2\left(mol\right)\Rightarrow m_{O_2}=38,4\left(g\right)\)
\(b,PTHH:C+O_2\underrightarrow{t}CO_2\)
\(n_C=2,5\left(mol\right)\Rightarrow n_{O_2}=2,5\left(mol\right)\Rightarrow m_{O_2}=80\left(g\right)\)
\(c,PTHH:4Al+3O_2\underrightarrow{t}2Al_2O_3\)
\(n_{Al}=2,5\left(mol\right)\Rightarrow n_{O_2}=1,875\left(mol\right)\Rightarrow m_{O_2}=60\left(g\right)\)
\(d,PTHH:2H_2+O_2\underrightarrow{t}2H_2O\)
\(TH_1:\left(đktc\right)n_{H_2}=1,5\left(mol\right)\Rightarrow n_{O_2}=0,75\left(mol\right)\Rightarrow m_{O_2}=24\left(g\right)\)
\(TH_2:\left(đkt\right)n_{H_2}=1,4\left(mol\right)\Rightarrow n_{O_2}=0,7\left(mol\right)\Rightarrow m_{O_2}=22,4\left(g\right)\)
\(5,PTHH:S+O_2\underrightarrow{t}SO_2\)
\(n_{O_2}=0,46875\left(mol\right)\)
\(n_{SO_2}=0,3\left(mol\right)\)
Vì\(0,46875>0,3\left(n_{O_2}>n_{SO_2}\right)\)nên S phản ứng hết, bài toán tính theo S.
\(a,\Rightarrow n_S=n_{SO_2}=0,3\left(mol\right)\Rightarrow m_S=9,6\left(g\right)\)
\(n_{O_2}\left(dư\right)=0,16875\left(mol\right)\Rightarrow m_{O_2}\left(dư\right)=5,4\left(g\right)\)
\(6,a,PTHH:C+O_2\underrightarrow{t}CO_2\)
\(n_{O_2}=1,5\left(mol\right)\Rightarrow n_C=1,5\left(mol\right)\Rightarrow m_C=18\left(g\right)\)
\(b,PTHH:2H_2+O_2\underrightarrow{t}2H_2O\)
\(n_{O_2}=1,5\left(mol\right)\Rightarrow n_{H_2}=0,75\left(mol\right)\Rightarrow m_{H_2}=1,5\left(g\right)\)
\(c,PTHH:S+O_2\underrightarrow{t}SO_2\)
\(n_{O_2}=1,5\left(mol\right)\Rightarrow n_S=1,5\left(mol\right)\Rightarrow m_S=48\left(g\right)\)
\(d,PTHH:4P+5O_2\underrightarrow{t}2P_2O_5\)
\(n_{O_2}=1,5\left(mol\right)\Rightarrow n_P=1,2\left(mol\right)\Rightarrow m_P=37,2\left(g\right)\)
\(7,n_{O_2}=5\left(mol\right)\Rightarrow V_{O_2}=112\left(l\right)\left(đktc\right)\);\(V_{O_2}=120\left(l\right)\left(đkt\right)\)
\(8,PTHH:C+O_2\underrightarrow{t}CO_2\)
\(m_C=0,96\left(kg\right)\Rightarrow n_C=0,08\left(kmol\right)=80\left(mol\right)\Rightarrow n_{O_2}=80\left(mol\right)\Rightarrow V_{O_2}=1792\left(l\right)\)
\(9,n_p=0,2\left(mol\right);n_{O_2}=0,3\left(mol\right)\)
\(PTHH:4P+5O_2\underrightarrow{t}2P_2O_5\)
Vì\(\frac{0,2}{4}< \frac{0,3}{5}\)nên P hết O2 dư, bài toán tính theo P.
\(a,n_{O_2}\left(dư\right)=0,05\left(mol\right)\Rightarrow m_{O_2}\left(dư\right)=1,6\left(g\right)\)
\(b,n_{P_2O_5}=0,1\left(mol\right)\Rightarrow m_{P_2O_5}=14,2\left(g\right)\)
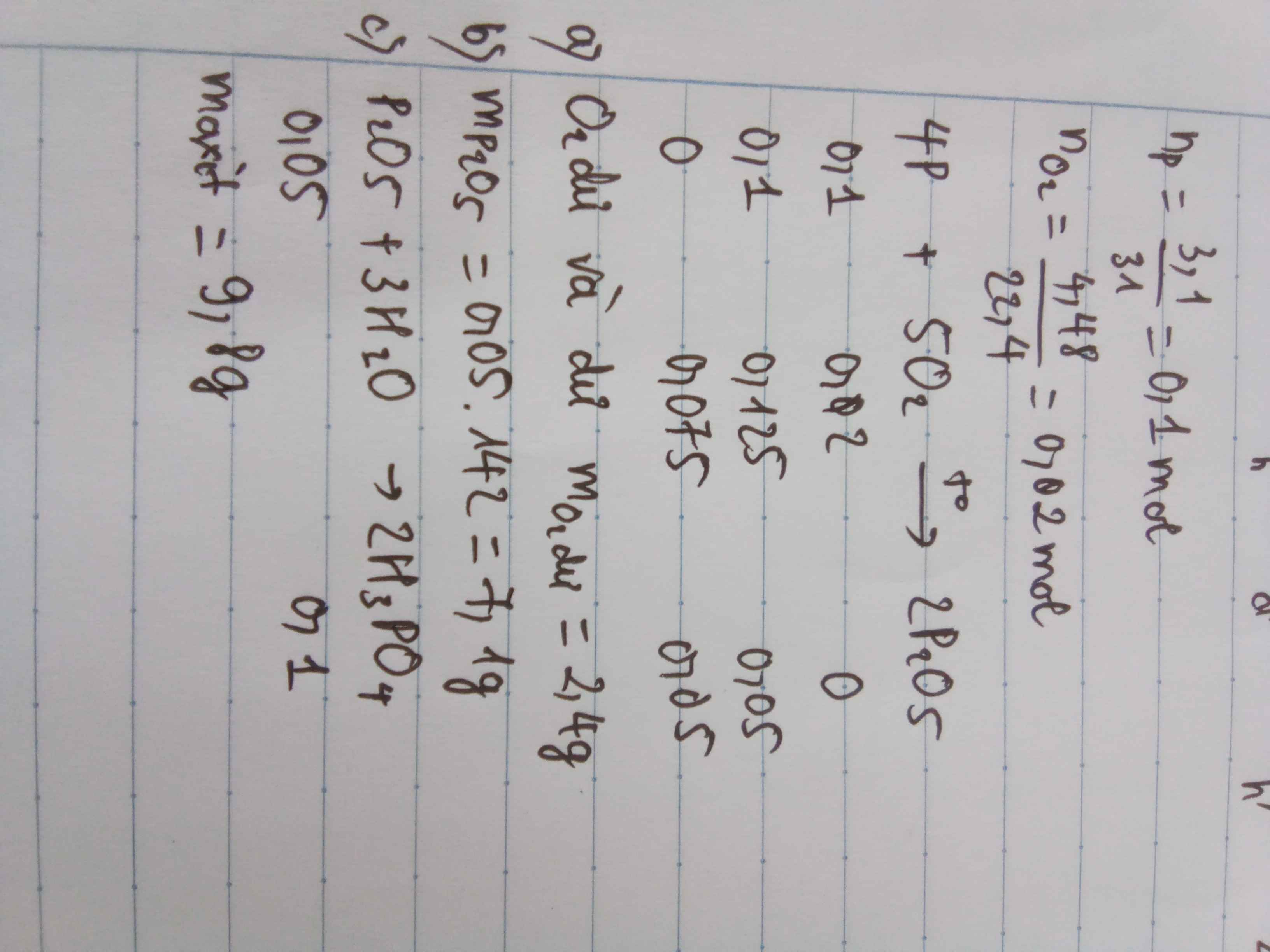
\(a) n_P = \dfrac{18,6}{31} = 0,6(mol)\\ n_{O_2} = \dfrac{20,16}{22,4} = 0,9(mol)\\ 4P + 5O_2 \xrightarrow{t^o} 2P_2O_5\\ \dfrac{n_P}{4} = 0,15 < \dfrac{n_{O_2}}{5} = 0,18 \to O_2\ dư\\ n_{O_2\ pư} = \dfrac{5}{4}n_P = 0,75(mol)\\ \Rightarrow m_{O_2\ dư} = (0,9-0,75).32 = 4,8(gam)\\ b) n_{Fe} = \dfrac{56}{56} = 1(mol)\)
\(3Fe + 2O_2 \xrightarrow{t^o} Fe_3O_4\\ \dfrac{n_{Fe}}{3} = \dfrac{1}{3}<\dfrac{n_{O_2}}{2} = 0,45\to Fe\ cháy\ hết.\\ c)\ 2KMnO_4 \xrightarrow{t^o} K_2MnO_4 + MnO_2 + O_2\\ n_{KMnO_4} = 2n_{O_2} = 0,9.2 = 1,8(mol)\\ \Rightarrow m_{KMnO_4} = 1,8.158 =284,4(gam)\)