Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

a, PT: \(CaCO_3+2HCl\rightarrow CaCl_2+CO_2+H_2O\)
b, Có: \(n_{CO_2}=0,02\left(mol\right)\)
Theo PT: \(n_{CaCO_3}=n_{CO_2}=0,02\left(mol\right)\)
\(\Rightarrow\left\{{}\begin{matrix}\%m_{CaCO_3}=\dfrac{0,02.100}{5}.100\%=40\%\\\%m_{CaSO_4}=60\%\end{matrix}\right.\)
c, Có: \(n_{HCl\left(banđau\right)}=0,2.0,25=0,05\left(mol\right)\)
Theo PT: \(n_{HCl\left(pư\right)}=2n_{CO_2}=0,04\left(mol\right)\)
\(\Rightarrow n_{HCl\left(dư\right)}=0,01\left(mol\right)\)
\(\Rightarrow C_{M_{HCl\left(dư\right)}}=\dfrac{0,01}{0,2}=0,05M\)
Bạn tham khảo nhé!

a, Theo ĐLBTKL ta có: \(m_{O_2}=28,4-15,6=12,8\left(g\right)\Rightarrow n_{O_2}=\dfrac{12,8}{32}=0,4\left(mol\right)\)
PTHH: 4Al + 3O2 ---to→ 2Al2O3
Mol: x 0,75x
PTHH: 2Mg + O2 ---to→ 2MgO
Mol: y 0,5y
Ta có: \(\left\{{}\begin{matrix}27x+24y=15,6\\0,75x+0,5y=0,4\end{matrix}\right.\Leftrightarrow\left\{{}\begin{matrix}x=0,4\\y=0,2\end{matrix}\right.\)
\(m_{Al}=0,4.27=10,8\left(g\right)\Rightarrow\%m_{Al}=\dfrac{10,8.100\%}{15,6}=69,23\%\)
\(m_{Mg}=15,6-10,8=4,8\left(g\right)\Rightarrow\%m_{Mg}=\dfrac{4,8.100\%}{15,6}=30,77\%\)
b, \(V_{O_2}=0,4.22,4=8,96\left(l\right)\)

\(n_{Cl_2}=a\left(mol\right)\)
\(n_{Mg}=b\left(mol\right)\)
\(n_X=a+b=\dfrac{7.84}{22.4}=0.35\left(mol\right)\left(1\right)\)
Bảo toàn khối lượng :
\(m_{Cl_2}+m_{O_2}=30.1-11.1=19\left(g\right)\)
\(\Leftrightarrow71a+32b=19\left(2\right)\)
\(\left(1\right),\left(2\right):a=0.2,b=0.15\)
\(Đặt:\)
\(n_{Mg}=x\left(mol\right),n_{Al}=y\left(mol\right)\)
\(m_Y=24x+27y=11.1\left(g\right)\left(3\right)\)
Bảo toàn e :
\(2x+3y=0.2\cdot2+0.15\cdot4=1\left(4\right)\)
\(\left(3\right),\left(4\right):x=0.35,y=0.1\)
\(\%Mg=\dfrac{0.35\cdot24}{11.1}\cdot100\%=75.67\%\)
\(\%Al=24.33\%\)

\(n_{Al}=a\left(mol\right),n_{Ag}=b\left(mol\right)\)
\(m_X=27a+108b=5.4\left(g\right)\left(1\right)\)
\(4Al+3O_2\underrightarrow{^{^{t^0}}}2Al_2O_3\)
\(a......0.75a...0.5a\)
\(m_{Cr}=0.5\cdot102a+108b=7.5\left(g\right)\left(2\right)\)
\(\left(1\right),\left(2\right):a=\dfrac{7}{80},b=\dfrac{9}{320}\)
\(V_{O_2}=0.75\cdot\dfrac{7}{80}\cdot22.4=1.47\left(l\right)\)
\(\%Al=\dfrac{\dfrac{7}{80}\cdot27}{5.4}\cdot100\%=43.75\%\)
\(\%Ag=56.25\%\)


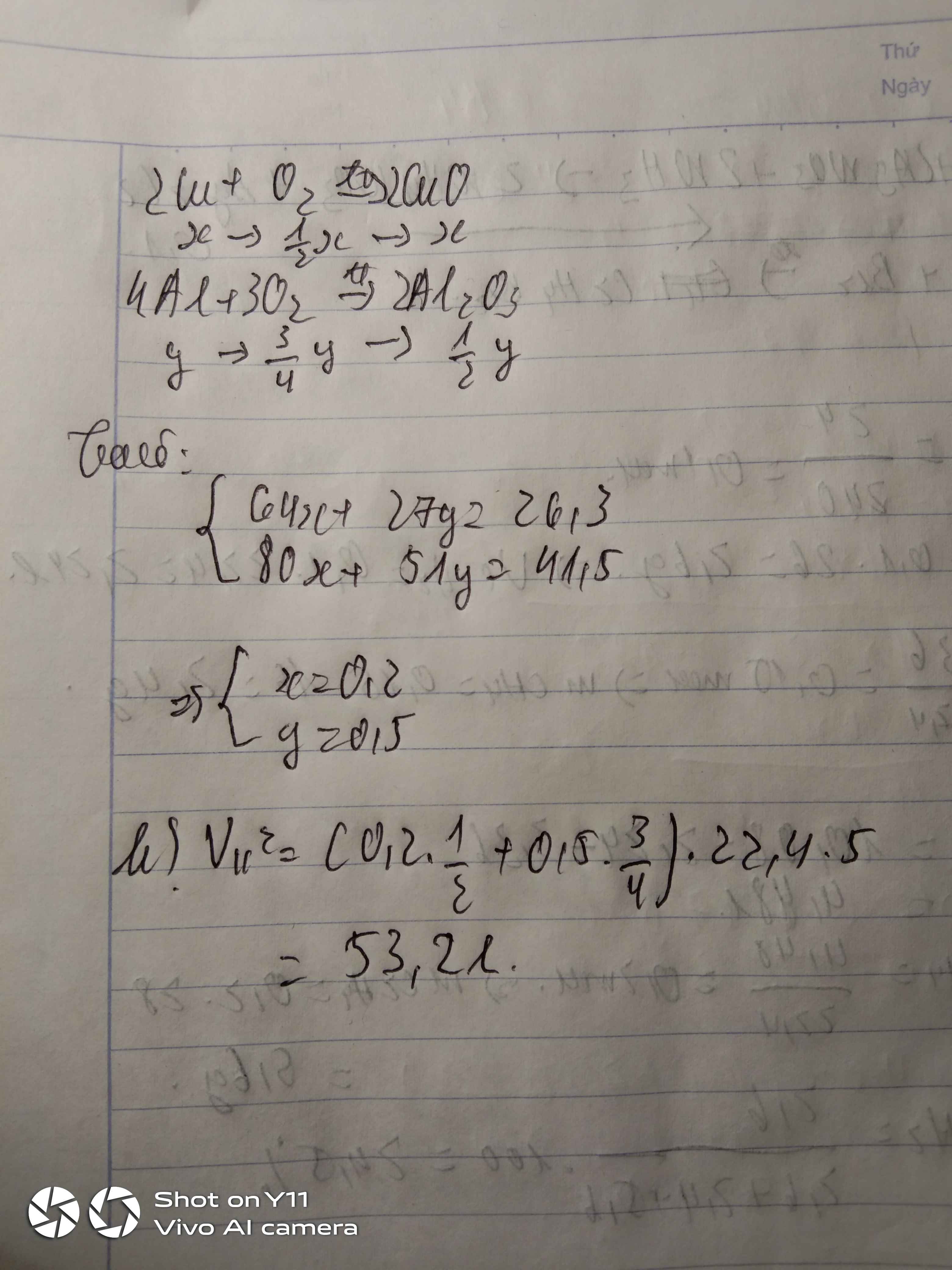
Gọi n Mg = x ( mol )
m Al = y ( mol )
-> 24x + 27 y = 7,5
PTHH :
\(2Mg+O_2\underrightarrow{t^o}2MgO\)
x 0,5x x
\(4Al+3O_2\underrightarrow{t^o}2Al_2O_3\)
y 3/4y 0,5y
-> \(0,5x+\dfrac{3}{4}y=n_{H_2}=\dfrac{4,48}{22,4}=0,2\left(mol\right)\)
Ta có Hệ Pt \(\left\{{}\begin{matrix}24x+27y=7,5\\0,5x+0,75y=0,2\end{matrix}\right.\)
Giải hệ PT , ta có :
x=0,05
y= 7/30
\(m_{Mg}=0,05.24=1,2\left(g\right)\)
\(m_{Al}=\dfrac{7}{30}.27=6,3\left(g\right)\)
\(m_{MgO}=0,05.40=2\left(g\right)\)
\(m_{Al_2O_3}=\dfrac{7}{30}.102:2=11,9\left(g\right)\)