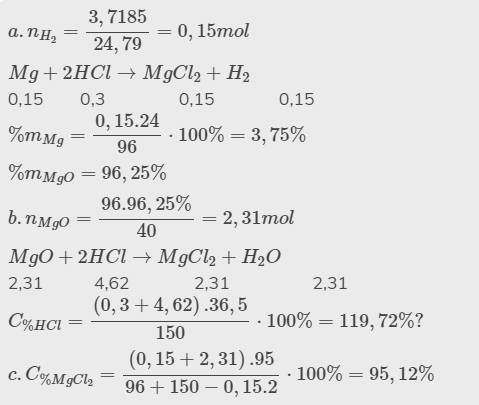
Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

CTHH: \(Mg+2HCl->MgCl_2+H_2\)(1). \(MgO+2HCl->MgCl_2+H_2O\)(2). Số MOL \(MgCl_2\) thu được: \(n_{MgCl_2}=\dfrac{28,5}{95}=0,3\left(mol\right)\) a) Gọi x,y lần lượt là số mol của Mg và MgO. Theo đề bài ta có: 24x+40y=8,8(*). Theo PTHH(1)(2) ta có: x+y=0,3(**). Giải (*)(**):\(\left\{{}\begin{matrix}24x+40y=8,8\\x+y=0,3\end{matrix}\right.\Rightarrow\left\{{}\begin{matrix}x=0,2\\y=0,1\end{matrix}\right.\) Khối lượng các chất: \(m_{Mg}=0,2.24=4,8\left(g\right)\) \(m_{MgO}=0,1.40=4\left(g\right)\) Thành phần %: \(\%m_{Mg}=\dfrac{4,8}{8,8}.100\%=54,5\%\)\(\%m_{MgO}=100\%-54,5\%=45,5\%\)
b)Theo PTHH(1): \(n_{HCl}=2n_{Mg}=2.0,2=0,4\left(mol\right)\) Theo PTHH(2):\(n_{HCl}=2n_{MgO}=2.0,1=0,2\left(mol\right)\) \(\Sigma n_{HCl\left(1+2\right)}=0,4+0,2=0,6\left(mol\right)\) Khối lượng HCl: \(m_{HCl}=0,6.36,5=21,9\left(g\right)\)khối lượng dd HCl: \(m_{d^2HCl}=\dfrac{21,9.100}{14,6}=150\left(g\right)\)

Bài 1:
\(n_{H_2SO_4}=\dfrac{300.19,6\%}{98}=0,6\left(mol\right);n_{Al}=\dfrac{2,7}{27}=0,1\left(mol\right)\\ PTHH:2Al+3H_2SO_4\rightarrow Al_2\left(SO_4\right)_3+3H_2\\ Vì:\dfrac{0,1}{2}< \dfrac{0,6}{3}\Rightarrow H_2SO_4dư\\ n_{Al_2\left(SO_4\right)_3}=\dfrac{3n_{Al}}{2}=\dfrac{3.0,1}{2}=0,15\left(mol\right)\\ a,m_{Al_2\left(SO_4\right)_3}=342.0,15=51,3\left(g\right)\\ b,m_{ddsau}=m_{Al}+m_{ddH_2SO_4}-m_{H_2}=2,7+300-\dfrac{3}{2}.0,1.2=302,4\left(g\right)\\ c,C\%_{ddAl_2\left(SO_4\right)_3}=\dfrac{51,3}{302,4}.100\%\approx16,964\%\\ n_{H_2SO_4\left(dư\right)}=0,6-\dfrac{3}{2}.0,1=0,45\left(mol\right)\\ C\%_{ddH_2SO_4\left(dư\right)}=\dfrac{0,45.98}{302,4}.100\%\approx14,583\%\)
Bài 2:
\(PTHH:2Al+6HCl\rightarrow2AlCl_3+3H_2\\ Mg+2HCl\rightarrow MgCl_2+H_2\\ Đặt:n_{Al}=a\left(mol\right);n_{Mg}=b\left(mol\right)\left(a,b>0\right)\\ Hpt:\left\{{}\begin{matrix}27a+24b=7,8\\1,5a+b=\dfrac{8,96}{22,4}=0,4\end{matrix}\right.\Leftrightarrow\left\{{}\begin{matrix}a=0,2\\b=0,1\end{matrix}\right.\\ \%m_{Al}=\dfrac{0,2.27}{7,8}.100\%\approx69,231\%\Rightarrow\%m_{Mg}\approx100\%-69,231\%\approx30,769\%\)

- Đặt \(\left\{{}\begin{matrix}n_{Al}=a\left(mol\right)\\n_{Mg}=b\left(mol\right)\end{matrix}\right.\Rightarrow27a+24b=10,2\left(1\right)\)
Khí thu được sau p/ứ là khí H2: \(n_{H_2}=\dfrac{11,2}{22,4}=0,5\left(mol\right)\)
\(2Al+6HCl\rightarrow2AlCl_3+3H_2\uparrow\)
2 3 (mol)
a 3/2 a (mol)
\(Mg+2HCl\rightarrow MgCl_2+H_2\uparrow\)
1 1 (mol)
b b (mol)
Từ hai PTHH trên ta có: \(\dfrac{3}{2}a+b=0,5\left(2\right)\)
\(\left(1\right),\left(2\right)\) ta có hệ: \(\left\{{}\begin{matrix}27a+24b=10,2\\\dfrac{3}{2}a+b=0,5\end{matrix}\right.\)
Giải ra ta có \(\left\{{}\begin{matrix}a=0,2\left(mol\right)\\b=0,2\left(mol\right)\end{matrix}\right.\)
a) \(\%Al=\dfrac{m_{Al}}{m_{hh}}.100\%=\dfrac{0,2.27}{10,2}.100\%\approx52,94\%\)
\(\%Mg=100\%-\%Al=100\%-52,94=47,06\%\)
b)
\(3H_2+Fe_2O_3\rightarrow^{t^0}2Fe+3H_2O\)
3 1 2 (mol)
0,5 1/6 1/3 (mol)
\(m_{Fe}=\dfrac{1}{3}.56=\dfrac{56}{3}\left(g\right)\)
\(m_{Fe_2O_3\left(pứ\right)}=\dfrac{1}{6}.160=\dfrac{80}{3}\left(g\right)\)
\(m_{Fe_2O_3\left(dư\right)}=60-m_{Fe}=60-\dfrac{56}{3}=\dfrac{124}{3}\left(g\right)\)
\(a=\dfrac{124}{3}+\dfrac{80}{3}=68\left(g\right)\)

a) 2Al + 6HCl \(\rightarrow\) 2AlCl3 + 3H2
Fe + 2HCl \(\rightarrow\) FeCl2 + H2
b) Gọi nAl = x, nFe = y
=> 27x + 56y = 11 (1)
Theo pt: \(\Sigma\)nH2 = 1,5x + y = \(\dfrac{8,96}{22,4}=0,4mol\)(2)
Từ 1 + 2 => x = 0,2 , y = 0,1
=> mAl = 0,2.27 = 5,4 => %mAl = \(\dfrac{5,4}{11}.100\%\approx49,09\%\)
%mFe = 100 - 49,09 = 50,91%
c) Theo pt: nHCl = 2nH2 = 0,8 mol
=> mHCl = 0,8 . 36,5 = 29,2g
=> \(m_{dd}\)HCl = 29,2 : 10% = 292g
d) mdd sau phản ứng = m A + mHCl = 11 + 292 = 303g
Theo pt: nAlCl3 = nAl = 0,2 mol => m AlCl3 = 26,7g
=> C%AlCl3 = \(\dfrac{26,7}{303}.100\%\) = 8,81%
tương tự nFeCl2 = 0,1 mol => C%FeCl2 = 4,19%

\(a.Na_2CO_3+2HCl\rightarrow2NaCl+CO_2+H_2O\\ n_{NaCl}=n_{HCl}=2.n_{CO_2}=2.\dfrac{448:1000}{22,4}=0,04\left(mol\right)\\ C_{MddHCl}=\dfrac{0,04}{0,02}=2\left(M\right)\\ b.m_{NaCl}=58,5.0,04=2,34\left(g\right)\\ c.m_{Na_2CO_3}=106.0,02=2,12\left(g\right)\\ \%m_{Na_2CO_3}=\dfrac{2,12}{5}.100=42,4\%\\ \%m_{NaCl}=100\%-42,4\%=57,6\%\)
Bài 16 :
\(n_{CO2}=\dfrac{0,448}{22,4}=0,02\left(mol\right)\)
Pt : \(Na_2CO_3+2HCl\rightarrow2NaCl+CO_2+H_2O|\)
1 2 2 1 1
0,02 0,04 0,04 0,02
a) \(n_{HCl}=\dfrac{0,02.2}{1}=0,04\left(mol\right)\)
20ml = 0,02l
\(C_{M_{HCl}}=\dfrac{0,04}{0,02}=2\left(M\right)\)
b) \(n_{NaCl}=\dfrac{0,02.2}{1}=0,04\left(mol\right)\)
⇒ \(m_{NaCl}=0,04.58,5=2,34\left(g\right)\)
c) \(n_{Na2CO3}=\dfrac{0,04.1}{2}=0,02\left(mol\right)\)
⇒ \(m_{Na2CO3}=0,02.106=2,12\left(g\right)\)
\(m_{NaCl}=5-2,12=2,88\left(g\right)\)
0/0Na2CO3 = \(\dfrac{2,12.100}{5}=42,4\)0/0
0/0NaCl = \(\dfrac{2,88.100}{5}=57,6\)0/0
Chúc bạn học tốt

a, \(Zn+2HCl\rightarrow ZnCl_2+H_2\)
Ta có: \(n_{Zn}=\dfrac{13}{65}=0,2\left(mol\right)\)
Theo PT: \(n_{ZnCl_2}=n_{H_2}=n_{Zn}=0,2\left(mol\right)\)
\(\Rightarrow V_{H_2}=0,2.24,79=4,958\left(l\right)\)
b, \(n_{HCl}=2n_{Zn}=0,4\left(mol\right)\Rightarrow V_{HCl}=\dfrac{0,4}{2}=0,2\left(l\right)\)
c, \(C_{M_{ZnCl_2}}=\dfrac{0,2}{0,2}=1\left(M\right)\)