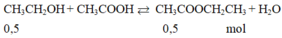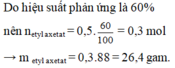Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

a, Ta có: \(n_{CH_3COOH}=\dfrac{9,6}{60}=0,16\left(mol\right)\)
PT: \(Mg+2CH_3COOH\rightarrow\left(CH_3COO\right)_2Mg+H_2\)
Theo PT: \(n_{\left(CH_3COO\right)_2Mg}=\dfrac{1}{2}n_{CH_3COOH}=0,08\left(mol\right)\)
\(\Rightarrow m_{\left(CH_3COO\right)_2Mg}=0,08.142=11,36\left(g\right)\)
b, PT: \(CH_3COOH+C_2H_5OH\underrightarrow{t^o,xt}CH_3COOC_2H_5+H_2O\)
Theo PT: \(n_{CH_3COOC_2H_5\left(LT\right)}=n_{CH_3COOH}=0,16\left(mol\right)\)
\(\Rightarrow m_{CH_3COOC_2H_5\left(LT\right)}=0,16.88=14,08\left(g\right)\)
Mà: thực tế thu được 10,56 (g)
\(\Rightarrow H\%=\dfrac{10,56}{14,08}.100\%=75\%\)

\(n_{Mg}=\dfrac{2,4}{24}=0,1\left(mol\right)\)
\(Mg+2CH_3COOH\rightarrow\left(CH_3COO\right)_2Mg+H_2\)
0,1 0,2
a. \(V_{CH_3COOH}=\dfrac{0,2}{1}=0,2\left(l\right)\)
b. \(CH_3COOH+C_2H_5OH⇌\left(H_2SO_{4đ},t^o\right)CH_3COOC_2H_5+H_2O\)
0,2 0,2
Với H% = 80
\(m_{CH_3COOC_2H_5}=\dfrac{0,2.88.80}{100}=14,08\left(g\right)\)

\(a)n_{CH_3COOH} = 0,2.2 = 0,4(mol)\\ Mg + 2CH_3COOH \to (CH_3COO)_2Mg + H_2\\ n_{Mg} = \dfrac{1}{2}n_{CH_3COOH} = 0,2(mol)\\ m_{Mg} = 0,2.24 = 4,8(gam)\\ b)\\ CH_3COOH + C_2H_5OH \buildrel{{H_2SO_4}}\over\rightleftharpoons CH_3COOC_2H_5 + H_2O\\ n_{CH_3COOH\ pư} = n_{este} = \dfrac{24,64}{88} = 0,28(mol)\\ H = \dfrac{0,28}{0,4}.100\% = 70\%\)

\(n_{CH_3COOC_2H_5}=\dfrac{4,4}{88}=0,05\left(mol\right)\)
PTHH: CH3COOH + C2H5OH --H2SO4(đ),to--> CH3COOC2H5 + H2O
0,05<--------------------------------------0,05
=> \(m_{CH_3COOH\left(lý.thuyết\right)}=0,05.60=3\left(g\right)\)
=> \(m_{CH_3COOH\left(tt\right)}=\dfrac{3.100}{60}=5\left(g\right)\)