Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

\(n_{H_2}=0.065\left(mol\right)\)
\(2H^++2e\rightarrow H_2\)
\(O_2+4e\rightarrow2O^{2-}\)
\(n_{O_2}=\dfrac{2\cdot0.065}{4}=0.0325\left(mol\right)\)
\(BTKL:\)
\(m_{oxit}=\dfrac{2.29}{2}+0.0325\cdot32=2.185\left(g\right)\)

a, Gọi \(\left\{{}\begin{matrix}n_{Fe}=a\left(mol\right)\\n_{Al}=b\left(mol\right)\end{matrix}\right.\)
\(n_{H_2}=\dfrac{11,2}{22,4}=0,5\left(mol\right)\)
PTHH:
Fe + 2HCl ---> FeCl2 + H2
a--->2a------------------>a
2Al + 6HCl ---> 2AlCl3 + 3H2
b---->3b-------------------->1,5b
=> \(\left\{{}\begin{matrix}56a+27b=16,6\\a+1,5b=0,5\end{matrix}\right.\Leftrightarrow a=b=0,2\left(mol\right)\)
=> \(\left\{{}\begin{matrix}m_{Fe}=0,2.56=11,2\left(g\right)\\m_{Al}=0,2.27=5,4\left(g\right)\end{matrix}\right.\)
b) \(C\%_{HCl}=\dfrac{\left(0,2.2+0,2.3\right).36,5}{300}.100\%=12,167\%\)
\(n_{H_2}=\dfrac{11,2}{22,4}=0,5\left(mol\right)\)
gọi nFe : a , nAl: b (a,b>0) => 56a + 27b = 16,6 (g)
\(pthh:Fe+2HCl\rightarrow FeCl_2+H_2\uparrow\)
a a
\(2Al+6HCl\rightarrow2AlCl_3+3H_2\uparrow\)
b \(\dfrac{3b}{2}\)
=> \(a+\dfrac{3b}{2}=0,5\)
ta có hệ pt
\(\left\{{}\begin{matrix}56a+27b=16,6\\a+\dfrac{3b}{2}=0,5\end{matrix}\right.\)
=> a= 0,2 , b = 0,2
\(\left\{{}\begin{matrix}m_{Fe}=0,2.56=11,2\left(g\right)\\m_{Al}=16,6-11,2=5,4\left(g\right)\end{matrix}\right.\)
\(pthh:Fe+2HCl\rightarrow FeCl_2+H_2\)
0,2 0,4
\(2Al+6HCl\rightarrow2AlCl_3+3H_2\)
0,2 0,6
=> \(m_{HCl}=\left(0,4+0,6\right).36,5=36,5\left(g\right)\)
=> \(C\%=\dfrac{36,5}{200}.100\%=18,25\%\)

Bài 1 :
Giả sử : hỗn hợp có 1 mol
\(n_{H_2}=a\left(mol\right),n_{O_2}=1-a\left(mol\right)\)
\(\overline{M_X}=0.3276\cdot29=9.5\left(\dfrac{g}{mol}\right)\)
\(\Rightarrow m_X=2a+32\cdot\left(1-a\right)=9.5\left(g\right)\)
\(\Rightarrow a=0.75\)
Cách 1 :
\(\%H_2=\dfrac{0.75}{1}\cdot100\%=75\%\)
\(\%O_2=100-75=25\%\)
Cách 2 em tính theo thể tích nhé !

Gọi \(n_{Zn}=a\left(mol\right)\rightarrow n_{Fe}=1,6a\left(mol\right)\)
Theo đề bài: \(65a+1,6a.56=7,73\rightarrow a=0,05\left(mol\right)\)
\(\rightarrow\left\{{}\begin{matrix}n_{Zn}=0,05\left(mol\right)\\n_{Fe}=0,05.1,6=0,08\left(mol\right)\end{matrix}\right.\)
PTHH:
Zn + 2HCl ---> ZnCl2 + H2
0,05 0,1 0,05 0,05
Fe + 2HCl ---> FeCl2 + H2
0,08 0,16 0,08 0,08
\(\rightarrow V_{H_2}=\left(0,05+0,08\right).22,4=2,912\left(l\right)\)
Gọi mE = a (g)
=> \(\left\{{}\begin{matrix}m_{Fe_2O_3}=48\%.a=0,48a\left(g\right)\\m_{CuO}=32\%.a=0,32a\left(g\right)\end{matrix}\right.\rightarrow\left\{{}\begin{matrix}n_{Fe_2O_3}=\dfrac{0,48a}{160}=0,003a\left(mol\right)\\n_{CuO}=\dfrac{0,32a}{80}=0,004a\left(mol\right)\end{matrix}\right.\)
PTHH:
Fe2O3 + 3H2 --to--> 2Fe + 3H2O
0,003a->0,009a
CuO + H2 --to--> Cu + H2O
0,004a->0,004a
\(\rightarrow0,13=0,004a+0,009a\\ \Leftrightarrow a=100\left(g\right)\)

2Zn+O2-to>2ZnO
0,1---0,05----0,1
n Zn=0,1 mol
nO2=0,025 mol
=>VO2=0,05.22,4=1,12l
=>mZnO=0,1.81=8,1g
c)Zn dư
=>m ZnO=0,05.81=4,05g
2Zn+O2-to>2ZnO
0,1---0,05----0,1
n Zn=6,5/65=0,1 mol
n O2=0,8/32=0,025 mol
=>VO2=0,05.22,4=1,12l
=>mZnO=0,1.81=8,1g
c)Zn dư
=>m ZnO=0,05.81=4,05g

\(n_{Zn}=\dfrac{m_{Zn}}{M_{Zn}}=\dfrac{19,5}{65}=0,3mol\)
\(Zn+\dfrac{1}{2}O_2\rightarrow\left(t^o\right)ZnO\)
1 1/2 1 (mol)
0,3 0,15 0,3 ( mol )
PƯ trên thuộc loại phản ứng hóa hợp
\(m_{ZnO}=n_{ZnO}.M_{ZnO}=0,3.81=24,3g\)
\(V_{O_2}=n_{O_2}.22,4=0,15.22,4=3,36l\)

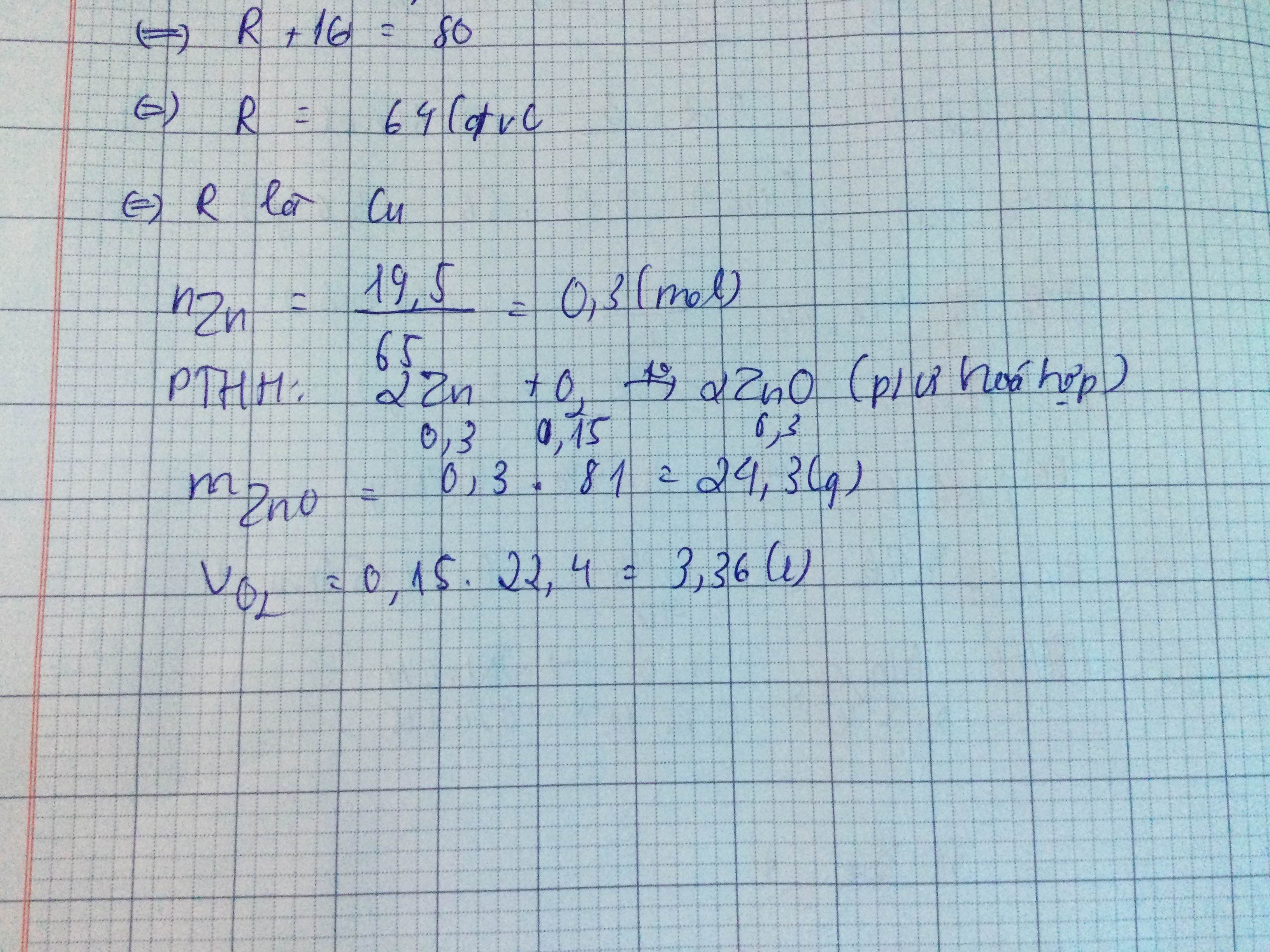
PTHH:
Phần 1:\(2CO+O_2\rightarrow2CO_2\) (1)
\(2H_2+O_2\rightarrow2H_2O\) (2)
\(CO_2+Ca\left(OH\right)_2\rightarrow CaCO_3\downarrow+H_2O\) (3)
Phần 2: \(CuO+H_2\rightarrow Cu+H_2O\) (4)
\(CuO+CO\rightarrow Cu+CO_2\) (5)
a, Ta có: \(n_{CaCO3}=\dfrac{20}{100}=0,2\left(mol\right)\)
Theo PTHH(3): \(n_{CO2\left(3\right)}=n_{CaCO3}=0,2\left(mol\right)\)
Theo PTHH(1): \(n_{CO2\left(1\right)}=n_{CO2\left(3\right)}=0,2\left(mol\right)\)
Vì 2 phần bằng nhau nên:
\(n_{CO\left(1\right)}=n_{CO\left(5\right)}=0,2\left(mol\right)\)
\(\Rightarrow\)Tổng \(n_{CO}=0,2\cdot2=0,4\left(mol\right)\)
Theo PTHH(5):\(n_{Cu}=n_{CO\left(5\right)}=0,2\left(mol\right)\)
\(\Rightarrow m_{Cu\left(5\right)}=0,2\cdot64=12,8\left(g\right)\)
\(\Rightarrow m_{Cu\left(4\right)}=19,2-12,8=6,4\left(g\right)\)
\(\Rightarrow n_{Cu\left(4\right)}=\dfrac{6,4}{64}=0,1\left(mol\right)\)
Theo PTHH(4): \(n_{H2}=n_{Cu}=0,1\left(mol\right)\)
\(\Rightarrow\)Tổng \(n_{H2}=0,1\cdot2=0,2\left(mol\right)\)
\(\Rightarrow n_{hh}=0,4+0,2=0,6\left(mol\right)\)
\(\Rightarrow V_{hh}=0,6\cdot22,4=13,44\left(l\right)\)
b, Vì tỉ lệ về thể tích cũng là tỉ lệ về số mol nên:
\(\Rightarrow\%V_{CO}=\dfrac{0,2}{0,6}\cdot100\%\approx33,33\%\) \(\%V_{H2}=100\%-33,33\%=66,67\%\)
\(\Rightarrow\%m_{CO}=\dfrac{0,2\cdot28}{0,2\cdot2+0,4\cdot28}\cdot100\%\approx96,55\%\)
\(\%m_{H2}=100\%-96,55\%=3,45\%\)