Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

Ta có: \(n_{N_2O}+n_{NO_2}+n_{N_2}=\dfrac{6,72}{22,4}=0,3\left(mol\right)\left(1\right)\)
BT e, có: 2nMg + 3nFe = 8nN2O + nNO2 + 10nN2
⇒ 8nN2O + nNO2 + 10nN2 = 1,9 (2)
Có: nHNO3 = 10nN2O + 2nNO2 + 12nN2 = 2,4.1 = 2,4 (mol) (3)
Từ (1), (2) và (3) \(\Rightarrow\left\{{}\begin{matrix}n_{N_2O}=0,1\left(mol\right)\\n_{NO_2}=0,1\left(mol\right)\\n_{N_2}=0,1\left(mol\right)\end{matrix}\right.\)
\(\Rightarrow\%V_{N_2}=\dfrac{0,1.22,4}{6,72.}100\%\approx33,33\%\)
\(n_{N_2O}=a\left(mol\right)\)
\(n_{NO_2}=b\left(mol\right)\)
\(n_{N_2}=c\left(mol\right)\)
\(\Rightarrow n_X=a+b+c=\dfrac{6.72}{22.4}=0.3\left(mol\right)\left(1\right)\)
Bảo toàn e :
\(8a+b+10c=0.65\cdot2+0.2\cdot3=1.9\left(2\right)\)
\(n_{H^+}=10a+2b+12c=2.4\left(mol\right)\left(3\right)\)
\(\left(1\right),\left(2\right),\left(3\right):a=b=c=0.1\left(mol\right)\)
\(\%V_{N_2}=\dfrac{0.1}{0.3}\cdot100\%=33.33\%\)

Ta có: \(n_{N_2O}+n_{NO_2}+n_{N_2}=\dfrac{11,2}{22,4}=0,5\left(mol\right)\left(1\right)\)
\(n_{HNO_3}=1,85.2=3,7\left(mol\right)\)
⇒ 10nN2O + 2nNO2 + 12nN2 = 3,7 (2)
\(n_{Mg}=\dfrac{16,8}{24}=0,7\left(mol\right)\)
\(n_{Fe}=\dfrac{28}{56}=0,5\left(mol\right)\)
BT e, có: 8nN2O + nNO2 + 10nN2 = 2nMg + 3nFe = 2,9 (3)
Từ (1), (2) và (3) \(\Rightarrow\left\{{}\begin{matrix}n_{N_2O}=0,15\left(mol\right)\\n_{NO_2}=0,2\left(mol\right)\\n_{N_2}=0,15\left(mol\right)\end{matrix}\right.\)
\(\Rightarrow\%V_{N_2}=\dfrac{0,15}{0,5}.100\%=30\%\)
m muối = mMg + mFe + 62.(8nN2O + nNO2 + 10nN2) = 224,6 (g)

Ta có: \(n_{NO}+n_{NO_2}+n_{N_2}=\dfrac{22,4}{22,4}=1\left(mol\right)\left(1\right)\)
Mà: mX = 35,8 (g)
\(\Rightarrow30n_{NO}+46n_{NO_2}+28n_{N_2}=35,8\left(2\right)\)
Có: \(n_{Al}=\dfrac{32,4}{27}=1,2\left(mol\right)\)
\(n_{Cu}=\dfrac{22,4}{64}=0,35\left(mol\right)\)
BT e, có: 3nNO + nNO2 + 10nN2 = 3nAl + 2nCu = 4,3 (3)
Từ (1), (2) và (3) \(\Rightarrow\left\{{}\begin{matrix}n_{NO}=0,3\left(mol\right)\\n_{NO_2}=0,4\left(mol\right)\\n_{N_2}=0,3\left(mol\right)\end{matrix}\right.\)
⇒ nHNO3 = 4nNO + 2nNO2 + 12nN2 = 5,6 (mol)

Đặt $n_{NO}=2a(mol);n_{NO_2}=a(mol)$
Bảo toàn e ta có: $6a+a=0,1.3+0,25.3\Rightarrow a=0,15(mol)$
Do đó $n_{A}=0,15.3=0,45(mol)\Rightarrow V_A=10,08(l)$
tham khảo trong:
https://moon.vn/hoi-dap/hoa-tan-hoan-toan-hon-hop-gom-01-mol-fe-va-025-mol-al-vao-dung-dich-hno3-du-thu-duoc-530914

Đáp án C.
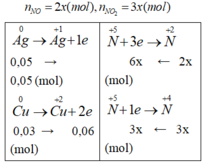
9x = 0,11; x= 11/900 => V = 5x.22,4 = 1,368 (l)

Ta có: \(n_{Fe}=\dfrac{5,6}{56}=0,1\left(mol\right)\)
\(n_{Mg}=\dfrac{2,4}{24}=0,1\left(mol\right)\)
Theo ĐLBT e, có: 3nFe + 2nMg = nNO2 ⇒ nNO2 = 0,5 (mol)
BTNT Fe, có: nFe(NO3)3 = nFe = 0,1 (mol)
BTNT Mg, có: nMg(NO3)2 = nMg = 0,1 (mol)
BTNT N, có: nHNO3 = 3nFe(NO3)3 + 2nMg(NO3)2 + nNO2 = 1 (mol)


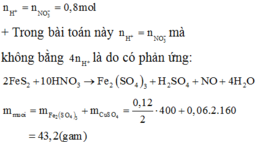


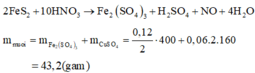
\(n_{HNO_3}=1.2\left(mol\right)\)
\(n_{NO}=a\left(mol\right)\)
\(n_{NO_2}=b\left(mol\right)\)
\(n_{N_2}=c\left(mol\right)\)
\(\Rightarrow a+b+c=\dfrac{5.6}{22.4}=0.25\left(1\right)\)
Bảo toàn e :
\(3\cdot0.2+1\cdot0.3=3a+b+10c\left(2\right)\)
\(n_{H^+}=4n_{NO}+2n_{NO_2}+12n_{N_2}\)
\(\Rightarrow4a+2b+12c=1.2\left(3\right)\)
\(\left(1\right),\left(2\right),\left(3\right):a=b=0.1,c=0.05\)
\(\%V_{NO_2}=\dfrac{0.1}{0.25}\cdot100\%=40\%\%0-\)