Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

\(C_4H_8 + H_2 \xrightarrow{t^o,Ni} C_4H_{10}\\ C_4H_{10}\xrightarrow{t^o,Ni} CH_4 + C_3H_6\\ 2CH_4 \xrightarrow{lln,1500^oc}C_2H_2 + 3H_2\\ 2C_2H_2 \xrightarrow{t^o,p,xt} C_4H_4\\ C_2H_2 + 2AgNO_3 + 2NH_3 \to C_2Ag_2 + 2NH_4NO_3\\ C_2H_2 + H_2\xrightarrow{t^o,PbCO_3} C_2H_4\)

\(BaCl_2+H_2SO_4\rightarrow BaSO_4+2HCl\)
\(x----x------x\)
\(n_{BaCl_2}=0,2\left(mol\right);n_{H_2SO_4}=0,125.0,4=0,05\left(mol\right)\)
Vậy H2SO4 phản ứng hết
\(\Rightarrow m_{BaSO_4}=0,05.233=11,65\left(g\right)\)

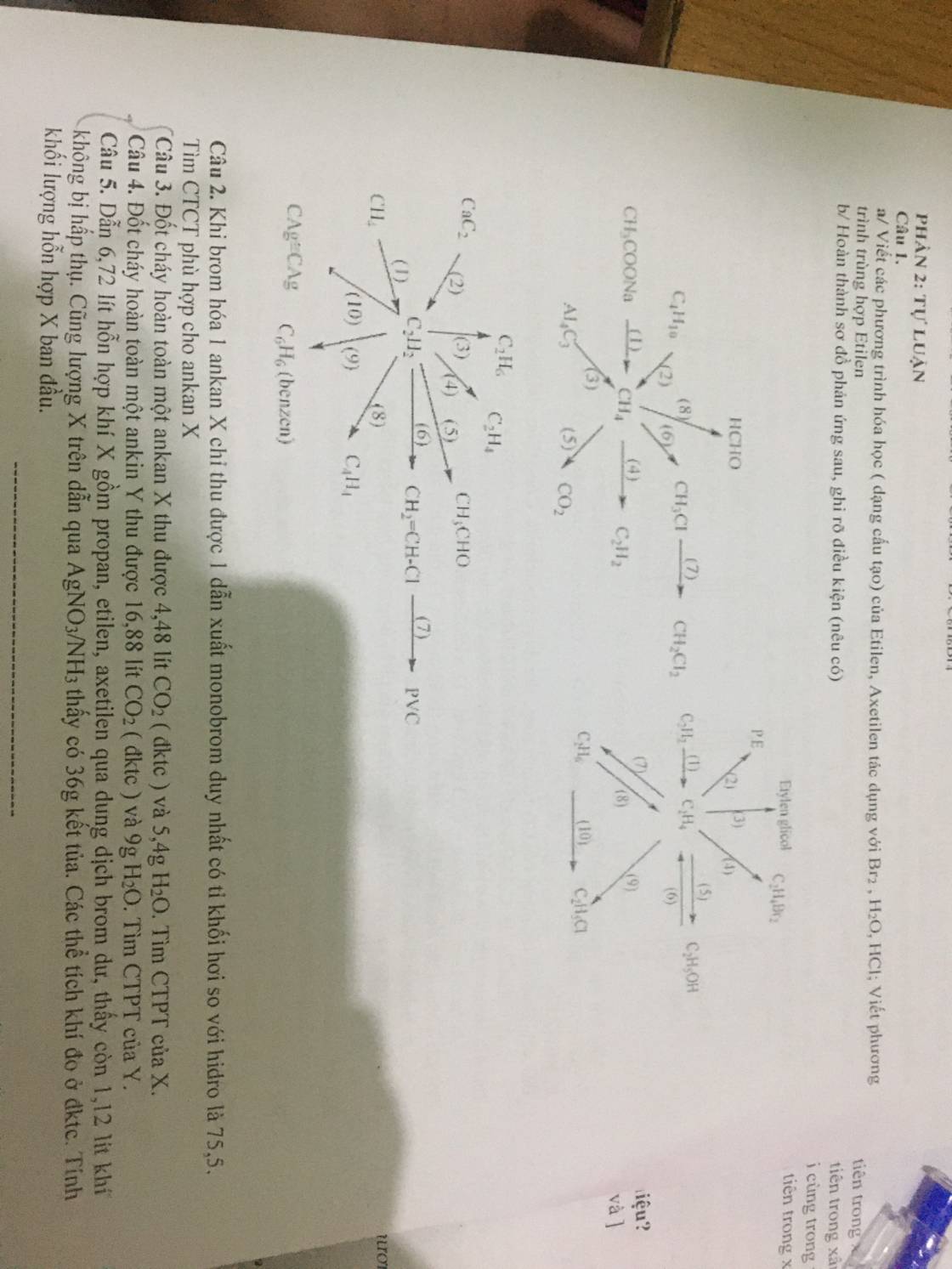
Câu 1:
\(2CH_4\underrightarrow{^{1500^oC,lln}}C_2H_2+3H_2\)
\(2CH\equiv CH\underrightarrow{t^o,xt}CH_2=CH-C\equiv CH\)
\(CH_2=CH-C\equiv CH+H_2\underrightarrow{t^o,Ni}CH_2=CH-CH=CH_2\)
\(nCH_2=CH-CH=CH_2\underrightarrow{^{t^o,p,xt}}\left(-CH_2-CH_2-CH_2-CH_2-\right)_n\)
Câu 2:
\(CH_3COONa+NaOH\underrightarrow{^{t^o,CaO}}CH_4+Na_2CO_3\)
\(2CH_4\underrightarrow{^{1500^oC,lln}}C_2H_2+3H_2\)
\(C_2H_2+H_2\underrightarrow{t^o,Pd}C_2H_4\)
\(C_2H_4+H_2O\underrightarrow{t^o,xt}C_2H_5OH\)

- Chuỗi 1:
(1) \(CH_3COONa+NaOH\underrightarrow{^{t^o,CaO}}CH_4+Na_2CO_3\)
(2) \(C_4H_{10}\underrightarrow{^{t^o,p,xt}}CH_4+C_3H_6\)
(3) \(Al_4C_3+12H_3O\rightarrow4Al\left(OH\right)_3+3CH_4\)
(4) \(2CH_4\underrightarrow{^{1500^oC,lln}}C_2H_2+3H_2\)
(5) \(CH_4+2O_2\underrightarrow{t^o}CO_2+2H_2O\)
(6) \(CH_4+Cl_2\underrightarrow{^{as}}CH_3Cl+HCl\)
(7) \(CH_3Cl+Cl_2\underrightarrow{^{as}}CH_2Cl_2+HCl\)
(8) \(CH_4+O_2\underrightarrow{^{t^o,xt}}HCHO+H_2O\)
- Chuỗi 2:
(1) \(C_2H_2+H_2\underrightarrow{^{t^o,Pd}}C_2H_4\)
(2) \(nCH_2=CH_2\underrightarrow{^{t^o,p,xt}}\left(-CH_2-CH_2-\right)_n\)
(3) \(3C_2H_4+2KMnO_4+4H_2O\rightarrow3C_2H_4\left(OH\right)_2+2MnO_{2\downarrow}+2KOH\)
(4) \(C_2H_4+Br_2\rightarrow C_2H_4Br_2\)
(5) \(C_2H_4+H_2O\underrightarrow{^{t^o,xt}}C_2H_5OH\)
(6) \(C_2H_5OH\xrightarrow[170^oC]{^{H_2SO_4\left(đ\right)}}C_2H_4+H_2O\)
(7) \(C_2H_4+H_2\underrightarrow{t^o,Ni}C_2H_6\)
(8) \(C_2H_6\underrightarrow{t^o,p,xt}C_2H_4+H_2\)
(9) \(C_2H_4+HCl\underrightarrow{t^o,xt}C_2H_5Cl\)
(10) \(C_2H_6+Cl_2\underrightarrow{as}C_2H_5Cl+HCl\)
- Chuỗi 3:
(1) \(2CH_4\underrightarrow{^{1500^oC,lln}}C_2H_2+3H_2\)
(2) \(CaC_2+2H_2O\rightarrow C_2H_2+Ca\left(OH\right)_2\)
(3) \(C_2H_2+2H_2\underrightarrow{^{t^o,Ni}}C_2H_6\)
(4) \(C_2H_2+H_2\underrightarrow{t^o,Pd}C_2H_4\)
(5) \(C_2H_2+H_2O\underrightarrow{t^o,xt}CH_3CHO\)
(6) \(C_2H_2+HCl\underrightarrow{t^o,xt}CH_2=CHCl\)
(7) \(nCH_2=CHCl\underrightarrow{t^o,p,xt}\left(-CH_2-CHCl-\right)_n\)
(8) \(2C_2H_2\underrightarrow{t^o,p,xt}C_4H_4\)
(9) \(3C_2H_2\underrightarrow{t^o,p,xt}C_6H_6\)
(10) \(C_2H_2+2AgNO_3+2NH_3\rightarrow Ag_2C_2+2NH_4NO_3\)

Ta có: \(n_{H_2O}=\dfrac{9}{18}=0,5\left(mol\right)\Rightarrow n_H=2n_{H_2O}=1\left(mol\right)\)
\(n_{CO_2}=\dfrac{17,6}{44}=0,4\left(mol\right)\Rightarrow n_C=n_{CO_2}=0,4\left(mol\right)\)
Đốt cháy A cũng là đốt cháy n - butan ban đầu.
BTKL, có: \(m=m_C+m_H=0,4.12+1.1=5,8\left(g\right)\)
Bạn tham khảo nhé!
2CH4 -> (t°, làm lạnh nhanh) C2H2 + 3H2
C2H2 + H2 -> (t°, xt, Pd) C2H4
C2H4 + HCl -> (askt) C2H5Cl
C2H5Cl + NaOH -> C2H5OH + NaCl
\(2CH_4\underrightarrow{t^o}C_2H_2+3H_2\)
\(C_2H_2\underrightarrow{t^o,xt,p}C_4H_4\)
\(C_4H_4+HCl\rightarrow C_2H_5Cl+C_2\)
\(C_2H_5Cl+NaOH\rightarrow C_2H_5OH+NaCl\)