Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

\(n_{Al}=\dfrac{5,4}{27}=0,2\left(mol\right)\\a, PTHH:4Al+3O_2\rightarrow\left(t^o\right)2Al_2O_3\\ b,n_{O_2}=\dfrac{3}{4}.n_{Al}=\dfrac{3.0,2}{4}=0,15\left(mol\right)\\ \Rightarrow V_{O_2\left(đktc\right)}=0,15.22,4=3,36\left(l\right)\\ c,2KMnO_4\rightarrow\left(t^o\right)K_2MnO_4+MnO_2+O_2\\ n_{KMnO_4}=2.n_{O_2}=2.0,15=0,3\left(mol\right)\\ \Rightarrow m_{KMnO_4}=158.0,3=47,4\left(g\right)\)

\(n_{Zn}=\dfrac{m_{Zn}}{M_{Zn}}=\dfrac{19,5}{65}=0,3mol\)
\(Zn+\dfrac{1}{2}O_2\rightarrow\left(t^o\right)ZnO\)
1 1/2 1 (mol)
0,3 0,15 0,3 ( mol )
PƯ trên thuộc loại phản ứng hóa hợp
\(m_{ZnO}=n_{ZnO}.M_{ZnO}=0,3.81=24,3g\)
\(V_{O_2}=n_{O_2}.22,4=0,15.22,4=3,36l\)

a, 3Fe + 2O2 -to-> Fe3O4
b, nFe = m/M = 16,8/56 = 0,3 (mol)
từ pthh ta có: \(n_{O_2}=\dfrac{0,3.2}{3}=0,2\left(mol\right)\)
=>\(V_{O_2}=n.22,4=0,2.22,4=4,48\left(l\right)\)
c, C1: từ pthh ta có: \(n_{Fe_3O_4}=\dfrac{0,3.1}{3}=0,1\left(mol\right)\)
=>\(m_{Fe_3O_4}=n.M=0,1.\left(56.3+4.16\right)=0,1.232=23,2\left(g\right)\)
C2: \(m_{O_2}=n.M=0,2.32=6,4\left(g\right)\)
Áp dụng ĐLBTKL ta co:
\(m_{Fe_3O_4}=m_{Fe}+m_{O_2}=16,8+6,4=23,2\left(g\right)\)

Ta có: \(n_P=\dfrac{6,2}{31}=0,2\left(mol\right)\)
a. \(PTHH:4P+5O_2\overset{t^o}{--->}2P_2O_5\)
Theo PT: \(n_{O_2}=\dfrac{5}{4}.n_P=\dfrac{5}{4}.0,2=0,25\left(mol\right)\)
\(\Rightarrow V_{O_2}=0,25.22,4=5,5\left(lít\right)\)
b. Theo PT: \(n_{P_2O_5}=\dfrac{1}{2}.n_P=\dfrac{1}{2}.0,2=0,1\left(mol\right)\)
\(\Rightarrow m_{P_2O_5}=0,1.142=14,2\left(g\right)\)
\(n_P=\dfrac{6,2}{31}=0,2\left(mol\right)\\ 4P+5O_2-^{t^o}\rightarrow2P_2O_5\\ a.n_{O_2}=\dfrac{5}{4}n_P=0,25\left(mol\right)\\ \Rightarrow m_{O_2}=0,25.32=8\left(g\right)\\ b.BTKLm_P+m_{O_2}=m_{P_2O_5}\\ \Rightarrow m_{P_2O_5}=6,2+8=14,2\left(g\right)\)

Bài 4 câu a đề là thể tích H2 nha bạn
a)\(Fe2O3+3H2-->2Fe+3H2O\)
\(n_{Fe2O3}=\frac{12}{160}=0,075\left(mol\right)\)
\(n_{H2}=3n_{Fe2O3}=0,225\left(mol\right)\)
\(V_{H2}=0,225.22,4=5,04\left(l\right)\)
b)\(n_{Fe}=2n_{Fe2O3}=0,15\left(mol\right)\)
\(m_{Fe}=0,15.56=8,4\left(g\right)\)
Bài 6
a)\(Zn+H2SO4-->ZnSO4+H2\)
\(n_{Zn}=\frac{19,5}{65}=0,3\left(mol\right)\)
\(n_{ZnSO4}=n_{Zn}=0,3\left(mol\right)\)
\(m_{ZnSO4}=0,3.162=48,3\left(g\right)\)
b)\(n_{H2}=n_{Zn}=0,3\left(mol\right)\)
\(V_{H2}=0,3.22,4=6,72\left(l\right)\)

a/ Ta có: \(n_{Mg}=\dfrac{4.8}{24}=0.2\left(mol\right)\)
PTHH:
\(2Mg+O_2\underrightarrow{t^o}2MgO\)
2 1
0.2 x
\(=>x=\dfrac{0.2\cdot1}{2}=0.1=n_{O_2}\)
\(=>V_{O_2\left(đktc\right)}=0.1\cdot22.4=2.24\left(l\right)\)
b/ \(2Mg+O_2\underrightarrow{t^o}2MgO\)
2 2
0.2 y
\(=>y=\left(0.2\cdot2\right):2=0.2=n_{MgO}\)
\(=>m_{MgO}=0.2\cdot\left(24+16\right)=8\left(g\right)\)

\(n_{Al}=\dfrac{5,4}{27}=0,2mol\)
\(4Al+3O_2\underrightarrow{t^o}2Al_2O_3\)
0,2 0,15 0,1
\(m_{Al_2O_3}=0,1\cdot102=10,2g\)
\(2KClO_3\underrightarrow{t^o}2KCl+3O_2\)
0,1 0,15
\(m_{KClO_3}=0,1\cdot122,5=12,25g\)
\(PTHH:4Al+3O_2\underrightarrow{t^o}2Al_2O_3\)
\(n_{Al}=5,4:27=0,2\left(mol\right)\)
\(\Rightarrow n_{Al_2O_3}=0,2.2:4=0,1\left(mol\right);n_{O_2}=0,2.3:4=0,15\left(mol\right)\)
\(m_{Al_2O_3}=0,1.102=10,2\left(g\right)\)
b)\(PTHH:2KClO_3\underrightarrow{t^o}2KCl+3O_2\)
\(n_{O_2}=0,15\left(mol\right)\)(câu a)
\(\Rightarrow n_{KClO_3}=0,15.2:3=0,1\left(mol\right)\)
\(m_{KClO_3}=0,1.123,5=12,35\left(g\right)\)

nFe= 2,8/56=0,05(mol)
PTHH: 3Fe+2O2-> Fe3O4
0,05 0,003 0,0167
a) mFe3O4= 0,0167. 232=3,8744(g)
b) Vo2 = 0,003.22,4=0,0672(l)
Ta có : nFe=2,8/56=0,05 mol
3Fe+2O2--->Fe3O4 (1)
a) theo pt (1) nFe3O4=1/3nFe=1/60 mol
=> mFe3O4=1/60 . MFe3O4 =58/15 gam
b) nO2 =2/3 nFe=0,05 .2/3=1/30 mol
=> VO2=1/30 .22,4=0,7467 lít
c) Vkhông khí=5. VO2=3,73 lít.
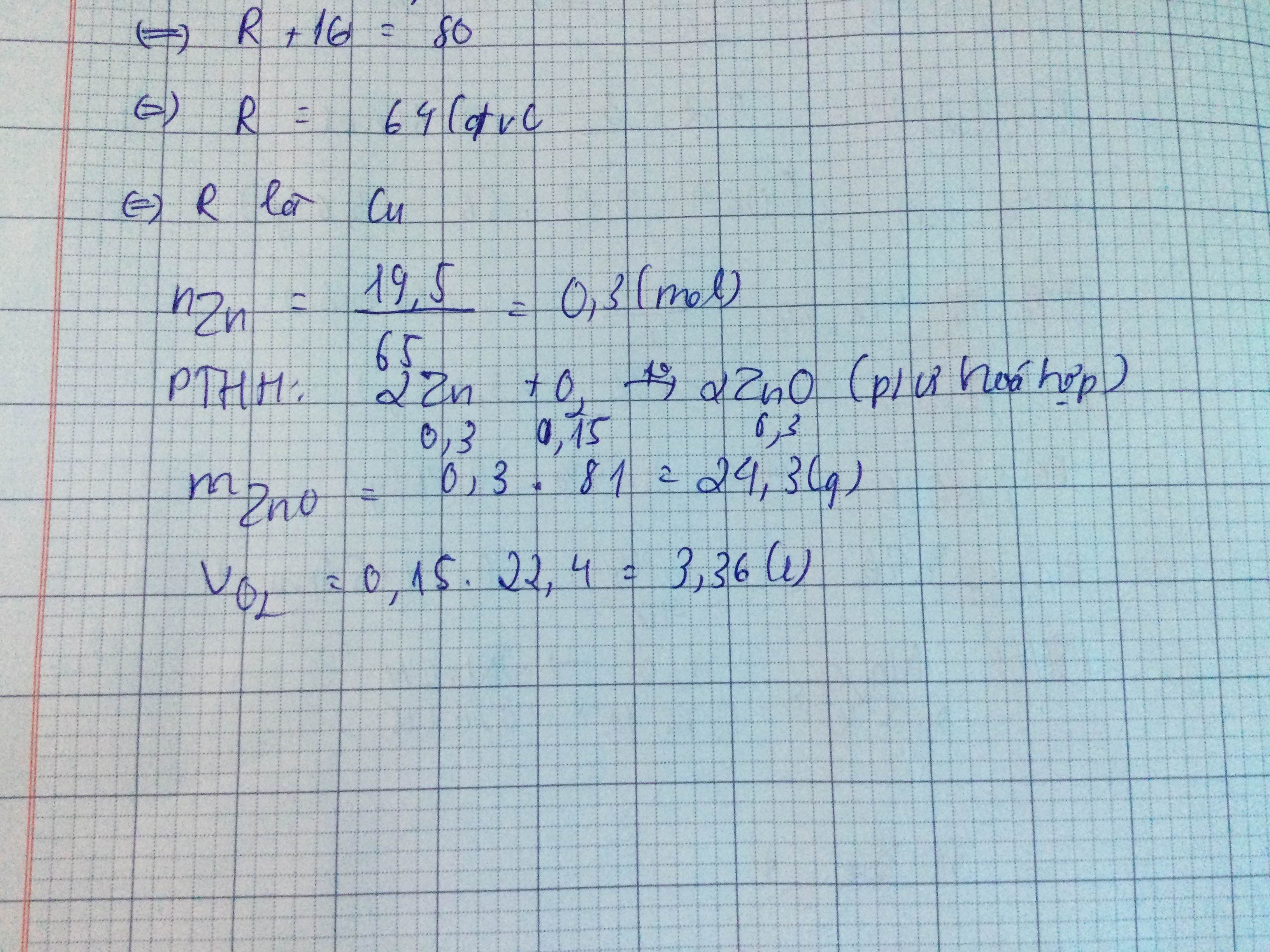
\(n_{KMnO4}=\dfrac{15,8}{158}=0,1\left(mol\right)\)
a) Pt : \(2KMnO_4\underrightarrow{t^o}K_2MnO_4+MnO_2+O_2|\)
2 1 1 1
0,1 0,05
b) \(n_{O2}=\dfrac{0,1.1}{2}=0,05\left(mol\right)\)
\(V_{O2\left(dktc\right)}=0,05.22,4=1,12\left(l\right)\)
c) \(n_{Fe}=\dfrac{5,6}{56}=0,1\left(mol\right)\)
Pt : \(3Fe+2O_2\underrightarrow{t^o}Fe_3O_4|\)
3 2 1
0,1 0,05 0,025
Lập tỉ số so sánh : \(\dfrac{0,1}{3}>\dfrac{0,05}{2}\)
⇒ Fe dư , O2 phản ứng hết
⇒ Tính toán dựa vào số mol của O2
\(n_{Fe3O4}=\dfrac{0,05.1}{2}=0,025\left(mol\right)\)
⇒ \(m_{Fe3O4}=0,025.232=5,8\left(g\right)\)
Chúc bạn học tốt
Thank bạn nha