Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

\(\text{a) 2HNO3+Ba(OH)2->Ba(NO3)2+2H2O}\)
\(\text{nBa(OH)2=100x25,65%/171=0,15(mol)}\)
V dd Ba(OH)2=100/1,25=80(ml)
\(\Rightarrow\text{CMBa(OH)2=0,15/0,08=1,875(M)}\)
\(\text{b) nHNO3=0,2.1,6=0,32(mol)}\)
=>nHNO3 dư=0,02(mol)
mdd spu=200x1,2+100=340(g)
\(\left\{{}\begin{matrix}\text{C%HNO3 dư=0,02x63/340x100=0,37%}\\\text{C%Ba(NO3)2=0,15x261/340=11,51% }\end{matrix}\right.\)

\(n_{Fe}=n_{H_2}=0,1\left(mol\right)\)
\(\Rightarrow m_{Fe}=5,6\left(g\right)\)
\(\Rightarrow m_{Fe_2O_3}=16\left(g\right)\)
\(\Rightarrow n_{Fe}=2n_{Fe_2O_3}=0,2\left(mol\right)=n_{FeCl_3}\)
Lại có : \(n_{HCl}=2n_{H_2}+3n_{FeCl_3}=0,8\left(mol\right)\)
\(\Rightarrow m_{ddHCl}=292\left(g\right)\)
\(\Rightarrow V=\dfrac{2920}{11}\left(ml\right)=\dfrac{73}{275}\left(l\right)\)
\(\Rightarrow C_{MFeCl_3}=\dfrac{0,2}{\dfrac{73}{275}}=\dfrac{55}{73}\left(M\right)\)

PTHH: \(Zn+2HCl\rightarrow ZnCl_2+H_2\uparrow\)
\(Al_2O_3+6HCl\rightarrow2AlCl_3+3H_2O\)
a) Ta có: \(n_{H_2}=\dfrac{8,96}{22,4}=0,4\left(mol\right)=n_{Zn}\)
\(\Rightarrow\%m_{Zn}=\dfrac{0,4\cdot65}{36,2}\cdot100\%\approx71,23\%\) \(\Rightarrow\%m_{Al_2O_3}=28,77\%\)
c) Ta có: \(n_{Al_2O_3}=\dfrac{36,2-0,4\cdot65}{102}=0,1\left(mol\right)\)
Theo PTHH: \(n_{HCl}=2n_{Zn}+6n_{Al_2O_3}=1,4\left(mol\right)\)
\(\Rightarrow m_{ddHCl}=\dfrac{1,4\cdot36,5}{10\%}=511\left(g\right)\) \(\Rightarrow V_{ddHCl}=\dfrac{511}{1,1}\approx464,5\left(ml\right)=0,4645\left(l\right)\)
c) Theo PTHH: \(\left\{{}\begin{matrix}n_{ZnCl_2}=0,4\left(mol\right)\\n_{AlCl_3}=0,2\left(mol\right)\end{matrix}\right.\) \(\Rightarrow\left\{{}\begin{matrix}C_{M_{ZnCl_2}}=\dfrac{0,4}{0,4645}\approx0,86\left(M\right)\\C_{M_{AlCl_3}}=\dfrac{0,2}{0,4645}\approx0,43\left(M\right)\end{matrix}\right.\)

Câu 3. Hòa tan 13,7 gam Ba trong 250ml H2O (D = 1,008 g/ml) thu được dung dịch X và khí Y (đktc)
a) Tính C% của dung dịch X.
b) Lấy 212,4 gam dung dịch X tác dụng với 14,7 gam dung dịch H2SO440% thu được dung dịch Z. Tìm C% các chất tan trong Z.
Giải :
\(a)n_{Ba}=\dfrac{13,7}{137}=0,1\left(mol\right)\\ Ba+H_2O\rightarrow Ba\left(OH\right)_2+H_2\\ n_{Ba\left(OH\right)_2}=n_{H_2}=n_{Ba}=0,1\left(mol\right)\\m_{H_2}=0,1.2=0,2\left(g\right)\\ m_{H_2O}=250.1,008=252\left(g\right)\\ m_{ddsaupu}=13,7+252-0,2=265,5\left(g\right)\\ C\%_{Ba\left(OH\right)_2}=\dfrac{0,1.171}{265,5}.100=\dfrac{380}{59\%}= 6,44\%\\b)n_{Ba\left(OH\right)_2}=\dfrac{212,4.\dfrac{380}{59}\%}{171} =0,08\left(mol\right)\)
\(n_{H_2SO_4}=\dfrac{14,7.40\%}{98}=0,06\left(mol\right)\\ Ba\left(OH\right)_2+H_2SO_4\rightarrow BaSO_4+2H_2O\\ Lậptỉlệ:\dfrac{0,08}{1}>\dfrac{0,06}{2}\Rightarrow Ba\left(OH\right)_2dư\\ n_{BaSO_4}=n_{H_2SO_4}=0,06\left(mol\right)\\ m_{ddsaupu}=212,4+14,7-0,06.233=213,12\left(g\right)\\ n_{Ba\left(OH\right)_2pư}=n_{H_2SO_4}=0,06\left(mol\right)\\ n_{Ba\left(OH\right)_2dư}=0,08-0,06=0,02\left(mol\right)\\ \Rightarrow C\%_{Ba\left(OH\right)_2dư}=\dfrac{0,02.171}{213,12}.100=1,61\%\)
5. \(Fe+H_2SO_4\rightarrow FeSO_4+H_2\\ Fe_2O_3+3H_2SO_4\rightarrow Fe_2\left(SO_4\right)_3+3H_2O\\ n_{H_2}=n_{Fe}=\dfrac{2,24}{22,4}=0,1\left(mol\right)\\ \Rightarrow\%m_{Fe}=\dfrac{0,1.56}{37,6}.100=14,89\%;\%m_{Fe_2O_3}=100-14,89=85,11\%\)

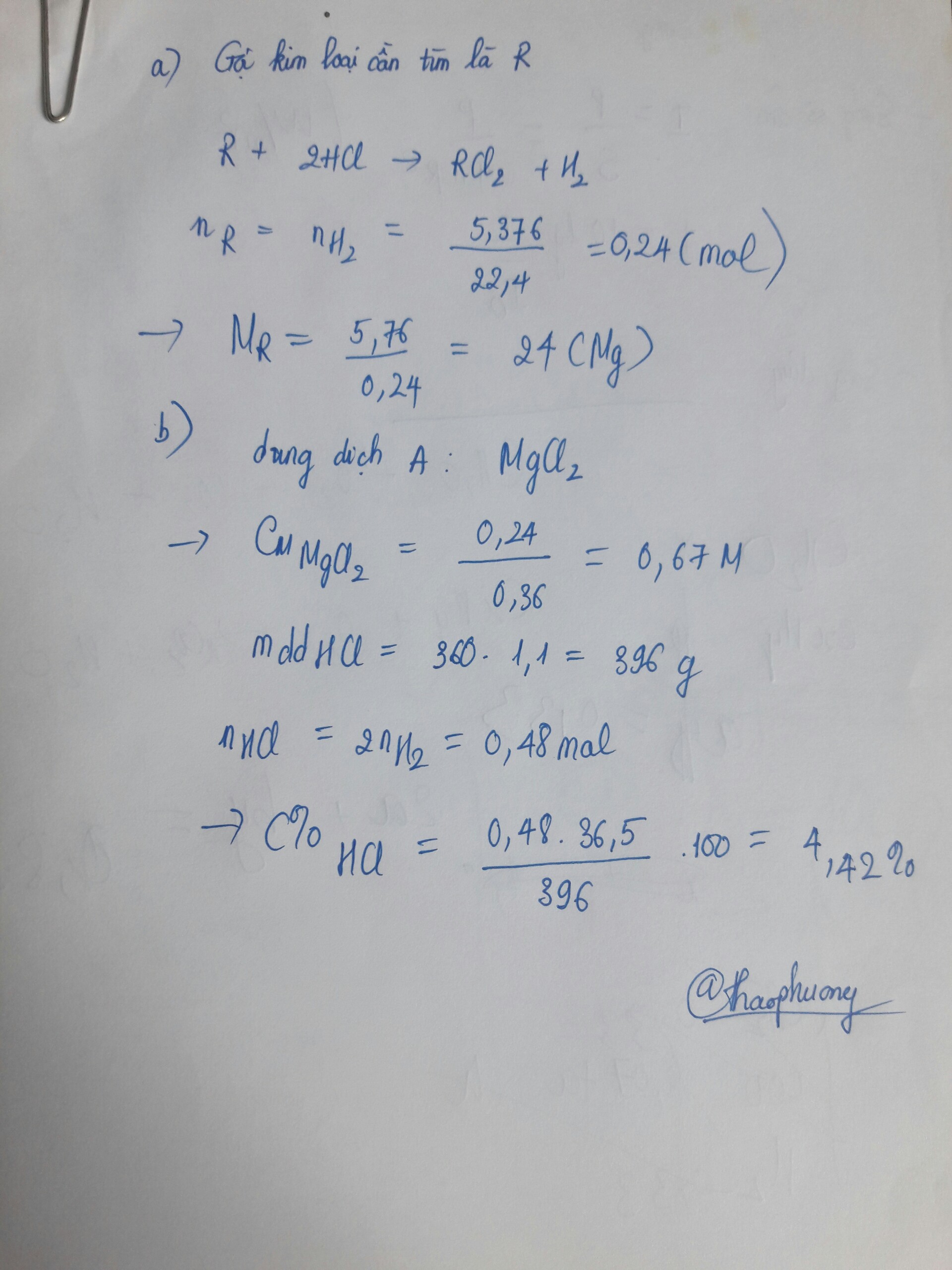
Đáp án A
=>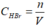 = 2,04M
= 2,04M