Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

PTHH: \(2KMnO_4\underrightarrow{t^o}K_2MnO_4+MnO_2+O_2\uparrow\)
a) Ta có: \(n_{KMnO_4}=\dfrac{94,8}{158}=0,6\left(mol\right)\)
\(\Rightarrow n_{O_2\left(lýthuyết\right)}=0,3\left(mol\right)\) \(\Rightarrow n_{O_2\left(thực\right)}=0,3\cdot90\%=0,27\left(mol\right)\)
\(\Rightarrow V_{O_2\left(thực\right)}=0,27\cdot22,4=6,048\left(l\right)\)
b) PTHH: \(4Al+3O_2\underrightarrow{t^o}2Al_2O_3\)
Theo PTHH: \(n_{Al_2O_3}=\dfrac{2}{3}n_{O_2}=0,18\left(mol\right)\)
\(\Rightarrow m_{Al_2O_3}=0,18\cdot102=18,36\left(g\right)\)

\(n_P=\dfrac{6,2}{31}=0,2\left(mol\right)\\ n_{O_2}=\dfrac{8,96}{22,4}=0,4\left(mol\right)\\ PTHH:4P+5O_2\underrightarrow{t^o}2P_2O_5\\ LTL:\dfrac{0,2}{4}< \dfrac{0,4}{5}\Rightarrow O_2dư\)
\(n_{O_2\left(pư\right)}=\dfrac{5}{4}n_P=\dfrac{5}{4}.0,2=0,25\left(mol\right)\\ n_{O_2\left(dư\right)}=0,4-0,25=0,15\left(mol\right)\)
\(n_{P_2O_5\left(lt\right)}=\dfrac{1}{2}n_P=\dfrac{1}{2}.0,2=0,1\left(mol\right)\\ m_{P_2O_5\left(lt\right)}=0,1.142=14,2\left(g\right)\\ m_{P_2O_5\left(tt\right)}=0,1.142.80\%=11,36\left(g\right)\)

a.\(n_{KMnO_4}=\dfrac{m}{M}=\dfrac{31,6}{158}=0,2mol\)
\(PTHH:2KMnO_4\underrightarrow{np}K_2MnO_4+MnO_2+O_2\)
2 1 1 1 ( mol )
0,2 0,1
\(V_{O_2}=n.22,4=0,1.22,4=2,24l\)
b.\(n_{Fe}=\dfrac{m}{M}=\dfrac{11,2}{56}=0,2mol\)
\(3Fe+2O_2\underrightarrow{t^o}Fe_3O_4\)
3 2 1 ( mol )
0,2 0,1
0,1 0,1 0,05 ( mol )
\(m_{Fe_3O_4}=n.M=0,05.232=11,6g\)

\(n_{KMnO_4}=\dfrac{15.8}{158}=0.1\left(mol\right)\)
\(2KMnO_4\underrightarrow{t^0}K_2MnO_4+MnO_2+O_2\)
\(n_{O_2}=\dfrac{0.1}{2}=0.05\left(mol\right)\)
\(V_{O_2}=0.05\cdot22.4=1.12\left(l\right)\)
\(b.\)
\(n_P=\dfrac{6.2}{31}=0.2\left(mol\right)\)
\(4P+5O_2\underrightarrow{t^0}2P_2O_5\)
\(4.........5\)
\(0.2........0.05\)
\(LTL:\dfrac{0.2}{4}>\dfrac{0.05}{5}\Rightarrow Pdư\)
\(m_{P\left(dư\right)}=\left(0.2-0.04\right)\cdot31=4.96\left(g\right)\)
PTHH:2KMnO4--- K2MnO4+MnO2 +O2
ADCT nKmno4=15,8/158=0,1 mol
a, theo pt có nO2/nKmno4= 1/2
nO2=0,05 mol
ADCT V=n*22,4
VO2=0,05*22,4 =1,12 l
b, PTHH: 5O2+4P---2P2O5
ADCTnP=6,2/31=0,2 mol
Theo pt
nO2/5=0,01 bé hơn nP/4=0,05
P dư
theo pt nP(pư)/nO2=4/5
nP(p/ư)=0,04 mol
nP(dư)=0,05-0,04 =0,01 mol
ADCT:m=n*M
mP(dư)=0,01*31=0,31g

\(n_{KMnO_4}=\dfrac{63,2}{158}=0,4\left(mol\right)\\
pthh:2KMnO_4\underrightarrow{t^o}K_2MnO_4+MnO_2+O_2\)
0,4 0,2
=> \(V_{O_2\left(lt\right)}=0,2.22,4=4,48\left(l\right)\\
V_{O_2\left(tt\right)}=\dfrac{90.4,48}{100}=4,032\left(l\right)\)

\(n_{Fe}=\dfrac{8,4}{56}=0,15\left(mol\right)\\ pthh:3Fe+2O_2\underrightarrow{t^o}Fe_3O_{\text{4}}\)
0,15 0,1 0,05
\(m_{Fe_2O_4}=0,05.232=11,6\left(g\right)\\
V_{O_2}=0,1.11,4=2,24\left(l\right)\\
pthh:2KMnO_4\underrightarrow{t^o}K_2MnO_4+MnO_2+O_2\uparrow\)
0,2 0,1
\(m_{KMnO_4}=0,2.158=31,6\left(g\right)\)
\(n_{Fe}=\dfrac{8,4}{56}=0,15\left(mol\right)\\ pthh:3Fe+2O_2\underrightarrow{t^o}Fe_3O_4\)
0,15 0,1 0,05
\(m_{Fe_3O_{\text{ 4}}}=0,05.232=11,6\left(g\right)\\ V_{O_2}=0,1.22,4=2,24\left(l\right)\\ pthh:2KMnO_4\underrightarrow{t^o}K_2MnO_4+MnO_2+O_2\)
0,1 0,05
\(m_{KMnO_4}=0,1.158=15,8\left(g\right)\)
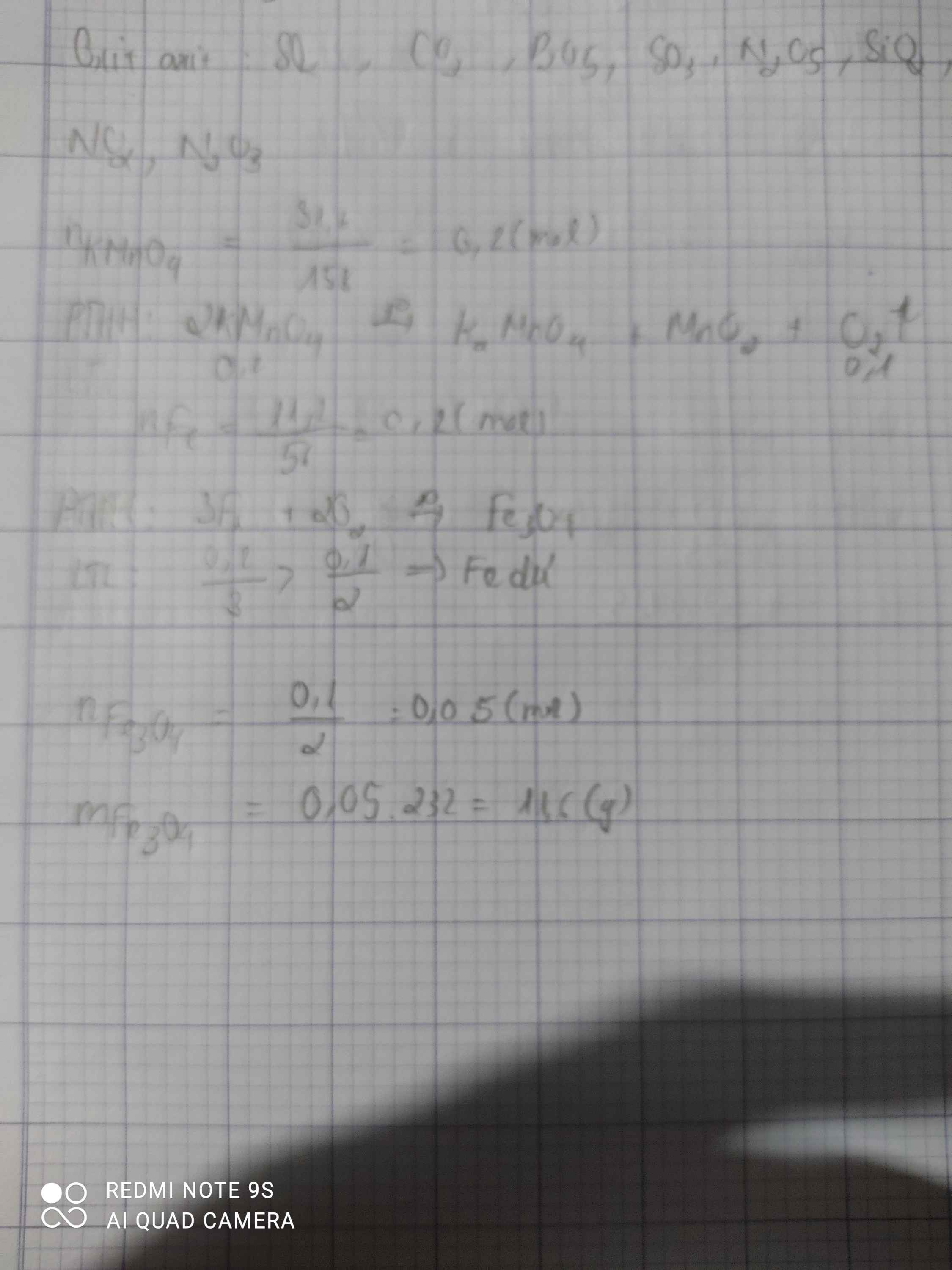

PT: \(2KMnO_4\underrightarrow{t^o}K_2MnO_4+MnO_2+O_2\)
Ta có: \(n_{KMnO_4}=\dfrac{31,6}{158}=0,2\left(mol\right)\)
Theo PT: \(n_{O_2\left(LT\right)}=\dfrac{1}{2}n_{KMnO_4}=0,1\left(mol\right)\)
Mà: H% = 80%
\(\Rightarrow n_{O_2\left(TT\right)}=0,1.80\%=0,08\left(mol\right)\)
\(\Rightarrow V_{O_2}=0,08.22,4=1,792\left(l\right)\)
PT: \(4P+5O_2\underrightarrow{t^o}2P_2O_5\)
Ta có: \(n_P=\dfrac{3,1}{31}=0,1\left(mol\right)\)
Xét tỉ lệ: \(\dfrac{0,1}{4}>\dfrac{0,08}{5}\), ta được P dư.
Theo PT: \(n_{P\left(pư\right)}=\dfrac{4}{5}n_{O_2}=0,064\left(mol\right)\)
\(\Rightarrow n_{P\left(dư\right)}=0,1-0,064=0,036\left(mol\right)\)
\(\Rightarrow m_{P\left(dư\right)}=0,036.31=1,116\left(g\right)\)
Bạn tham khảo nhé!