Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

a)
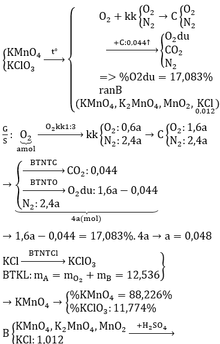
\(2KMnO_4\xrightarrow[]{t^o}K_2MnO_4+MnO_2+O_2\) (1)
\(2KClO_3\xrightarrow[]{t^o}2KCl+3O_2\) (2)
\(n_{KCl}=\dfrac{0,894}{74,5}=0,012\left(mol\right);m_B=\dfrac{0,894}{8,132\%}=11\left(g\right)\)
Gọi \(n_{O_2\left(sinh.ra\right)}=a\left(mol\right)\Rightarrow n_{kk}=3a\left(mol\right)\)
\(\Rightarrow\left\{{}\begin{matrix}n_{N_2}=3a.80\%=2,4a\left(mol\right)\\n_{O_2}=a+\left(3a-2,4a\right)=1,6a\left(mol\right)\end{matrix}\right.\)
\(n_C=\dfrac{0,528}{12}=0,044\left(mol\right)\)
\(C+O_2\xrightarrow[]{t^o}CO_2\) (3)
Vì hỗn hợp D gồm 3 khí và O2 chiếm 17,083%
\(\Rightarrow D:CO_2,O_{2\left(d\text{ư}\right)},N_2\)
BTNT C: \(n_{CO_2}=n_C=0,044\left(mol\right)\)
BTNT O: \(n_{O_2\left(d\text{ư}\right)}=n_{O_2\left(b\text{đ}\right)}-n_{CO_2}=1,6a-0,044\left(mol\right)\)
\(\Rightarrow\%V_{O_2}=\%n_{O_2}=\dfrac{1,6a-0,044}{1,6a-0,044+0,044+2,4a}.100\%=17,083\%\)
\(\Leftrightarrow a=0,048\left(mol\right)\left(TM\right)\)
ĐLBTKL: \(m_A=m_B+m_{O_2}=11+0,048.32=12,536\left(g\right)\)
Theo PT (2): \(n_{KClO_3}=n_{KCl}=0,012\left(mol\right)\)
\(\Rightarrow\left\{{}\begin{matrix}\%m_{KClO_3}=\dfrac{0,012.122,5}{12,536}.100\%=11,63\%\\\%m_{KMnO_4}=100\%-11,63\%=88,37\%\end{matrix}\right.\)
b) Theo PT (2): \(n_{O_2}=\dfrac{1}{2}n_{KMnO_4\left(p\text{ư}\right)}+\dfrac{3}{2}n_{KClO_3}\)
\(\Rightarrow n_{KMnO_4\left(p\text{ư}\right)}=2.\left(0,048-\dfrac{3}{2}.0,012\right)=0,06\left(mol\right)\)
\(n_{KMnO_4\left(b\text{đ}\right)}=\dfrac{12,536-0,012.122,5}{158}=0,07\left(mol\right)\)
\(\Rightarrow n_{KMnO_4\left(d\text{ư}\right)}=0,07-0,06=0,01\left(mol\right)\)
\(n_{KCl}=\dfrac{74,5}{74,5}+0,012=1,012\left(mol\right)\)
Theo PT (1): \(n_{K_2MnO_4}=n_{MnO_2}=\dfrac{1}{2}.n_{KMnO_4\left(p\text{ư}\right)}=0,03\left(mol\right)\)
PTHH:
\(2KMnO_4+10KCl+8H_2SO_4\rightarrow6K_2SO_4+2MnSO_4+5Cl_2+8H_2O\) (4)
\(K_2MnO_4+4KCl+4H_2SO_4\rightarrow3K_2SO_4+MnSO_4+2Cl_2+4H_2O\) (5)
\(MnO_2+2KCl+2H_2SO_4\rightarrow MnSO_4+K_2SO_4+Cl_2+2H_2O\) (6)
\(2KCl+H_2SO_4\xrightarrow[]{t^o}K_2SO_4+2HCl\) (7)
Theo PT (4), (5), (6): \(n_{KCl\left(p\text{ư}\right)}=5n_{KMnO_4\left(d\text{ư}\right)}+4n_{K_2MnO_4}+2n_{MnO_2}=0,23\left(mol\right)< 1,012\left(mol\right)=n_{KCl\left(b\text{đ}\right)}\)
`=> KCl` dư
Theo PT (4), (5), (6): \(n_{Cl_2}=\dfrac{1}{2}.n_{KCl\left(p\text{ư}\right)}=0,115\left(mol\right)\)
\(\Rightarrow V_{kh\text{í}}=V_{Cl_2}=0,115.22,4=2,576\left(l\right)\)

\(a/n_{Fe}=\dfrac{2,52}{56}=0,045mol\\ 3Fe+2O_2\xrightarrow[]{t^0}Fe_3O_4\\ n_{O_2}=\dfrac{0,045.2}{3}=0,03mol\\ V_{O_2}=0,03.22,4=0,672l\\ b/2KClO_3\xrightarrow[]{t^0}2KCl+3O_2\\ n_{KClO_3}=\dfrac{0,03.2}{3}=0,02mol\\ m_{KClO_3}=0,02.122,5=2,45g\)

\(n_{BaCO_3}=\dfrac{19,7}{197}=0,1\left(mol\right)\\ a,PTHH:BaCO_3+2CH_3COOH\rightarrow\left(CH_3COO\right)_2Ba+CO_2+H_2O\\ n_{CO_2}=n_{\left(CH_3COO\right)_2Ba}=n_{BaCO_3}=0,1\left(mol\right)\\ b,V_{CO_2\left(đktc\right)}=0,1.22,4=2,24\left(l\right)\\ c,m_{\left(CH_3COO\right)_2Ba}=255.0,1=25,5\left(g\right)\\ d,C_2H_5OH+O_2\rightarrow\left(men.giấm\right)CH_3COOH+H_2O\\ n_{CH_3COOH}=0,1.2=0,2\left(mol\right);n_{C_2H_5OH\left(LT\right)}=n_{CH_3COOH}=0,2\left(mol\right)\\ n_{C_2H_5OH\left(TT\right)}=0,2.75\%=0,15\left(mol\right)\\ m_{C_2H_5OH\left(TT\right)}=0,15.46=6,9\left(g\right)\)

PT: \(2KClO_3\underrightarrow{t^o}2KCl+3O_2\) (1)
\(2KMnO_4\underrightarrow{t^o}K_2MnO_4+MnO_2+O_2\) (2)
\(C+O_2\underrightarrow{t^o}CO_2\) (3)
Ta có: \(n_{KCl}=\dfrac{1,49}{74,5}=0,02\left(mol\right)\)
\(n_C=\dfrac{0,24}{12}=0,02\left(mol\right)\)
\(m_Y=\dfrac{1,49}{17,028\%}=8,75\left(g\right)\), Y gồm: KCl, KMnO4 (dư), K2MnO4, MnO2.
Theo PT (3): \(n_{CO_2}=n_{O_2\left(pư\right)}=n_C=0,02\left(mol\right)\)
\(\Rightarrow n_{O_2\left(dư\right)}=\dfrac{0,02}{40\%}-0,02=0,03\left(mol\right)\)
⇒ ΣnO2 = 0,02 + 0,03 = 0,05 (mol)
Theo PT (1): \(n_{O_2\left(1\right)}=\dfrac{3}{2}n_{KCl}=0,03\left(mol\right)\)
\(\Rightarrow n_{O_2\left(2\right)}=0,05-n_{O_2\left(1\right)}=0,02\left(mol\right)\)
Theo PT (2): \(n_{K_2MnO_4}=n_{MnO_2}=n_{O_2\left(2\right)}=0,02\left(mol\right)\)
\(n_{KMnO_4\left(pư\right)}=2n_{O_2}=0,04\left(mol\right)\)
Mà: mKCl + mKMnO4 (dư) + mK2MnO4 + mMnO2 = 8,75
⇒ mKMnO4 (dư) = 1,58 (g) \(\Rightarrow n_{KMnO_4\left(dư\right)}=\dfrac{1,58}{158}=0,01\left(mol\right)\)
\(\Rightarrow H\%=\dfrac{0,04}{0,04+0,01}.100\%=80\%\)

a,\(n_{CH_4}=\dfrac{32}{16}=2\left(mol\right)\)
PTHH: CH4 + 2O2 --to→ CO2 + 2H2O
Mol: 2 1 2
\(\Rightarrow m_{CO_2}=2.44=88\left(g\right)\)
b,\(V_{O_2}=1.24,79=24,79\left(l\right)\)

a) $n_{Al} = \dfrac{5,4}{27} = 0,2(mol)$
$4Al + 3O_2 \xrightarrow{t^o} 2Al_2O_3$
$n_{O_2} = \dfrac{3}{4}n_{Al} = 0,15(mol)$
$V_{O_2} = 0,15.22,4 = 3,36(lít)$
b) $2KClO_3 \xrightarrow{t^o,MnO_2} 2KCl + 3O_2$
$n_{KClO_3\ pư} = \dfrac{2}{3}n_{O_2} = 0,1(mol)$
$m_{KClO_3\ pư} = 0,1.122,5 = 12,25(gam)$
$\Rightarrow m_{KClO_3\ đã\ dùng} = 12,25 : (100\% - 10\%) = 13,61(gam)$

\(n_{CaCO_3}=\dfrac{20}{100}=0,2\left(mol\right)\\ a,PTHH:CaCO_3+2CH_3COOH\rightarrow\left(CH_3COO\right)_2Ca+CO_2+H_2O\\ b,n_{CO_2}=n_{\left(CH_3COO\right)_2Ca}=n_{CaCO_3}=0,2\left(mol\right)\\ b,V_{CO_2\left(đktc\right)}=0,2.22,4=4,48\left(l\right)\\ c,m_{\left(CH_3COO\right)_2Ca}=0,2.158=31,6\left(g\right)\\ d,C_4H_{10}+\dfrac{5}{2}O_2\rightarrow2CH_3COOH+H_2O\\ n_{C_4H_{10}\left(LT\right)}=\dfrac{0,4}{2}=0,2\left(mol\right)\\ n_{C_4H_{10}\left(TT\right)}=0,2:50\%=0,4\left(mol\right)\\ m_{C_4H_{10}\left(tt\right)}=58.0,4=23,2\left(g\right)\)
a)
\(n_{KClO_3}=\dfrac{24.5}{122.5}=0.2\left(mol\right)\)
\(2KClO_3\underrightarrow{^{^{t^0}}}2KCl+3O_2\)
\(n_{O_2}=\dfrac{3}{2}\cdot0.2=0.3\left(mol\right)\)
\(V_{O_2}=0.3\cdot22.4=6.72\left(l\right)\)
\(n_{O_2}=\dfrac{2.24}{22.4}=0.1\left(mol\right)\)
\(2KMnO_4\underrightarrow{^{^{t^0}}}K_2MnO_4+MnO_2+O_2\)
\(0.2...............................................0.1\)
\(n_{KMnO_4\left(bđ\right)}=\dfrac{0.2}{90\%}=\dfrac{2}{9}\left(mol\right)\)
\(m_{KMnO_4}=\dfrac{2}{9}\cdot158=35.11\left(g\right)\)