Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

\(nCH_2=CH_2\rightarrow\left(-CH_2-CH_2-\right)_n\)
\(CH_4\underrightarrow{^{H2,1400oC}}C_2H_2\rightarrow H_2C=CH-C\equiv CH\underrightarrow{^{+H2}}H_2C=\)
\(CH-CH=CH_2\underrightarrow{^{to,xt}}-\left(-CH_2-CH=CH-CH_2-\right)_n-\)

\(CaCO_3-^{t^o}\rightarrow CaO+CO_2\\ CaO+3C-^{t^o}\rightarrow CaC_2+CO\\ CaC_2+2H_2O\rightarrow C_2H_2+Ca\left(OH\right)_2\\2 C_2H_2-^{t^o,xt}\rightarrow CH\equiv C-CH=CH_2\\ CH\equiv C-CH=CH_2+H_2-^{t^o,Pd/PbCO_3}\rightarrow CH_2=CH-CH=CH_2\\ nCH_2=CH-CH=CH_2-^{t^o,p,xt}\rightarrow\left(-CH_2CH=CH-CH_2-\right)_n\)
\(a.nCH_2=CH_2-^{t^o,p,xt}\rightarrow\left(-CH_2-CH_2-\right)_n\\ b.nCH_2=C\left(CH_3\right)_2-^{t^o,p,xt}\rightarrow\left(-CH_2-C\left(CH_3\right)_2-\right)_n\\ c.nCH_2=CHCl-^{t^o,p,xt}\rightarrow\left(-CH_2-CHCl-\right)_n\\ d.nCH_2=CH-CH=CH_2-^{t^o,p,xt}\rightarrow\left(-CH_2CH=CH-CH_2-\right)_n\\ e.nCH_2-C\left(CH_3\right)-CH=CH_2-^{t^o,p,xt}\rightarrow\left(-CH_2-C\left(CH_3\right)=CH-CH_2-\right)_n\\ \)

\(2CH_4 \xrightarrow{1500^o,làm\ lạnh\ nhanh} C_2H_2 + 3H_2\\ 2C_2H_2 \xrightarrow{t^o,xt,p} C_4H_4\\ C_4H_4 + H_2 \xrightarrow{t^o,xt} CH_2=CH-CH=CH_2\\ nCH_2=CH-CH=CH_2 \xrightarrow{t^o,p,xt} (-CH_2-CH=CH-CH_2-)_n\)
\(2CH_4\underrightarrow{^{1500^0C},lln}C_2H_2+3H_2\)
\(2CH\equiv CH\underrightarrow{^{t^0,p,xt}}CH\equiv C-CH=CH_2\)
\(CH\equiv C-CH=CH_2+H_2\underrightarrow{^{\dfrac{Pd}{PbCO_3},t^0}}CH_2=CH-CH=CH_2\)
\(nCH_2=CH-CH=CH_2\underrightarrow{^{t^0,p,xt}}\left(-CH_2-CH=CH-CH_2-\right)_n\)

a) CH3COONa + NaOH\(\xrightarrow[t]{CaO}\) CH4 + Na2CO3
CH4+ Cl2 -> CHCl3 +HCl
b) C4H10 \(\xrightarrow[xt]{t}C_3H_6+CH_4\)
CH4 + O2-> CO2 +H2O
CO2 +Ca(OH)2-> CaCO3 +H2O
C) Al4C3 + H2O -> Al(OH)3 + CH4
CH4 \(\xrightarrow[lamlanhnhanh]{1500^0}\) C2H2 +H2
p/s: làm tới đây thôi ,tự cân bằng

Đáp án A
Hướng dẫn
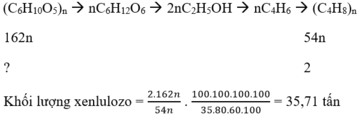
( C 6 H 10 O 5 ) n → n C 6 H 12 O 6 → 2 n C 2 H 5 O H → n C 4 H 6 → ( C 4 H 8 ) n 162 n 54 n ? 2
Khối lượng xenlulozo = 2 . 162 n 54 n . 100 . 100 . 100 . 100 35 . 80 . 60 . 100 = 35,71 tấn
\(2CH_4\xrightarrow[lln]{1500^0C}C_2H_2+3H_2\)
\(2C_2H_2\underrightarrow{t^0,p,xt}CH\equiv CH-CH_2=CH_2\)
\(CH\equiv CH-CH_2=CH_2+H_2\underrightarrow{PbCO_3,t^0}CH_2=CH-CH=CH_2\)
\(nCH_2=CH-CH=CH_2\underrightarrow{t^0,p,xt}\left(-CH_2-CH=CH-CH_2-\right)_n\)