Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

\(N_2+3H_2\underrightarrow{t^o,p}2NH_3\)
\(4NH_3+5O_2\underrightarrow{t^o,xt}4NO+6H_2O\)
\(2NO+O_2\rightarrow2NO_2\)
\(4NO_2+O_2+2H_2O\rightarrow4HNO_3\)
\(Cu+4HNO_3\rightarrow Cu\left(NO_3\right)_2+2NO_2+2H_2O\)
\(2Cu\left(NO_3\right)_2\underrightarrow{t^o}2CuO+4NO_2+O_2\)


Câu1.a)
1/Chất khử: NH3
Chất oxh : N2
2/Chất khử: Fe
Chất oxh : HNO3
b) 1/ \(QToxh:2N^{-3}\rightarrow\overset{0}{N_2}+6e|\times2\\ QTkhử:\overset{0}{O_2}+4e\rightarrow2O^{2-}|\times3\\ \Rightarrow4NH_3+3O_2\rightarrow2N_2+6H_2O\)
2/ \(QToxh:\overset{0}{Fe}\rightarrow Fe^{+3}+3e|\times1\\ QTkhử:N^{+5}+1e\rightarrow N^{+4}|\times3\\ \Rightarrow Fe+6HNO_3\rightarrow Fe\left(NO_3\right)_3+3NO_2+3H_2O\)
Câu 3. \(Đặt:\left\{{}\begin{matrix}n_{Fe}=x\left(mol\right)\\n_{Mg}=y\left(mol\right)\end{matrix}\right.\\ Fe+2HCl\rightarrow FeCl_2+H_2\\ Mg+2HCl\rightarrow MgCl_2\\ n_{H_2}=0,6\left(mol\right)\\ Tacó:\left\{{}\begin{matrix}x+y=0,6\left(mol\right)\\56x+24y=20,8\end{matrix}\right.\\ \Rightarrow\left\{{}\begin{matrix}x=0,2\\y=0,4\end{matrix}\right.\\ \Rightarrow\left\{{}\begin{matrix}m_{FeCl_2}=0,2.127=25,4\\m_{MgCl_2}=0,4.95=38\left(g\right)\end{matrix}\right.\)

Đáp án C
(1)NH3 + O2 ![]() NO + H2O
NO + H2O
(2)NH3 + 3CuO ![]() 3Cu + 3H2O + N2
3Cu + 3H2O + N2
(3)NH4NO3 + NaOH ![]() NaNO3 + NH3 + H2O
NaNO3 + NH3 + H2O
(4) NH4Cl ![]() NH3 + HCl
NH3 + HCl

Đáp án C
(1)NH3 + O2 → 850 ° , Pt NO + H2O
(2)NH3 + 3CuO → t ° 3Cu + 3H2O + N2
(3)NH4NO3 + NaOH → t ° NaNO3 + NH3 + H2O
(4) NH4Cl → t ° NH3 + HCl

Đáp án C
(1)NH3 + O2 → 850 ° , Pt NO + H2O
(2)NH3 + 3CuO → t ° 3Cu + 3H2O + N2
(3)NH4NO3 + NaOH → t ° NaNO3 + NH3 + H2O
(4) NH4Cl → t ° NH3 + HCl
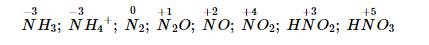
1/
\(\left(1\right)4NH_3+5O_2\xrightarrow[pt]{t^0}4NO+6H_2O\)
\(\left(2\right)NO+\frac{1}{2}O_2\rightarrow NO_2\)
\(\left(3\right)4NH_3+3O_2\underrightarrow{t^0}2N_2+6H_2O\)
\(\left(4\right)N_2+O_2\rightarrow2NO\uparrow\)
2/
\(\left(1\right)2NH_4NO_3\underrightarrow{t^0}2N_2+O_2+4H_2O\)
\(\left(2\right)N_2+3H_2\underrightarrow{\leftarrow}2NH_3\)
\(\left(3\right)3NH_3+FeCl_3+3H_2O\rightarrow Fe\left(OH\right)_3+3NH_4Cl\)
\(\left(4\right)Fe\left(OH\right)_3+3HNO_3\rightarrow Fe\left(NO_3\right)_3+3H_2O\)
\(\left(5\right)4Fe\left(NO_3\right)_3\rightarrow2Fe_2O_3+12NO_2+3O_2\)
\(\left(6\right)2NO_2+\frac{1}{2}O_2+H_2O\rightarrow2HNO_3\)
\(\left(7\right)NaOH+HNO_3\rightarrow NaNO_3+H_2O\)
\(\left(8\right)2NaNO_3\rightarrow2NaNO_2+O_2\)