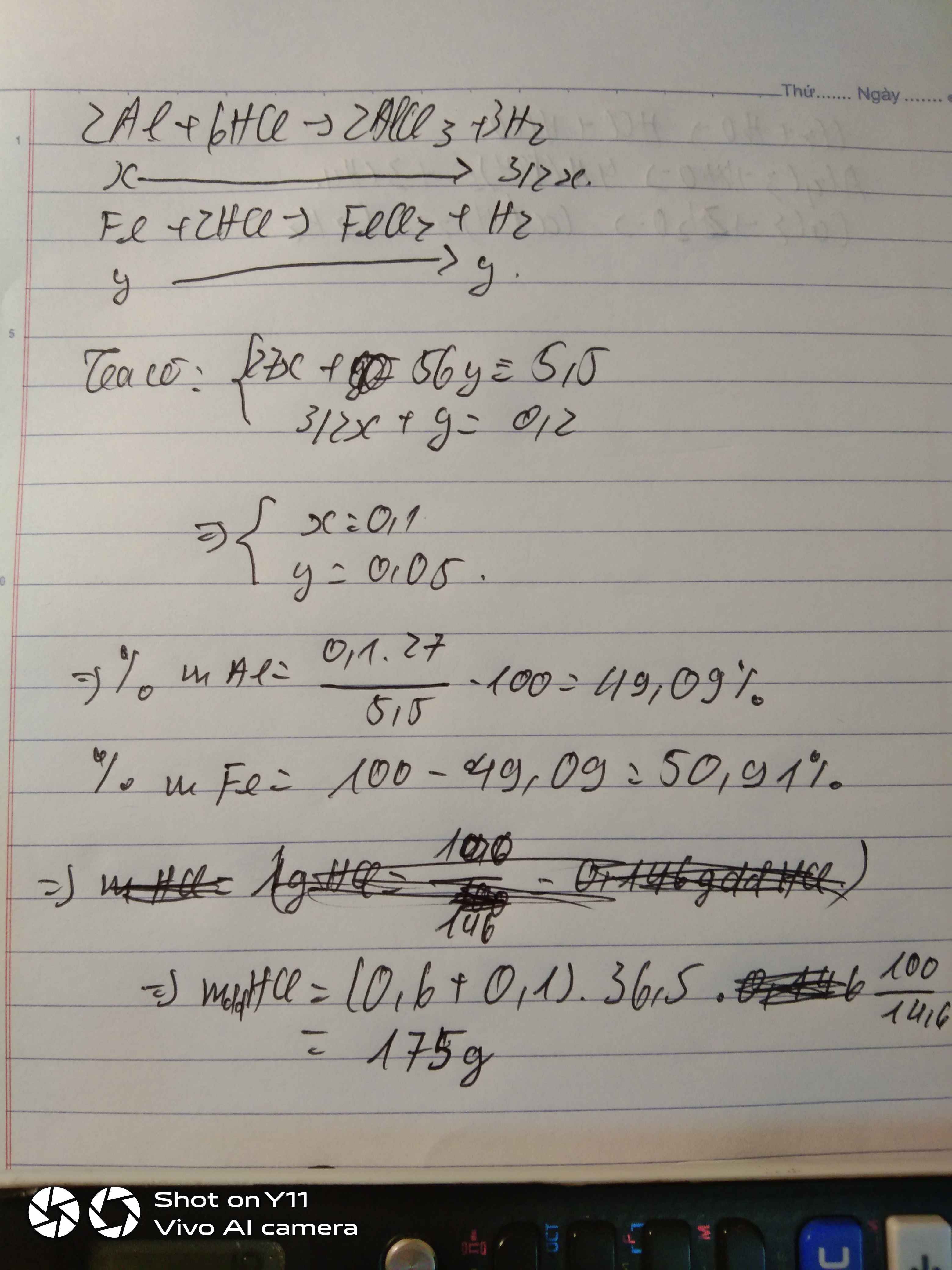Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

PTHH: \(2Mg+O_2\underrightarrow{t^o}2MgO\) (1)
\(4Al+3O_2\underrightarrow{t^o}2Al_2O_3\) (2)
a) Gọi số mol của Mg là a (mol) \(\Rightarrow n_{Al}=\dfrac{2}{3}a\left(mol\right)\)
\(\Rightarrow24a+27\cdot\dfrac{2}{3}a=6,3\) \(\Rightarrow a=0,15\left(mol\right)\)
\(\Rightarrow\left\{{}\begin{matrix}n_{MgO}=0,15\left(mol\right)\\n_{Al_2O_3}=0,05\left(mol\right)\end{matrix}\right.\) \(\Rightarrow\left\{{}\begin{matrix}m_{MgO}=0,15\cdot40=6\left(g\right)\\m_{Al_2O_3}=0,05\cdot102=5,1\left(g\right)\end{matrix}\right.\)
b) Theo các PTHH: \(\left\{{}\begin{matrix}n_{O_2\left(1\right)}=0,075\left(mol\right)\\n_{O_2\left(2\right)}=0,075\left(mol\right)\end{matrix}\right.\)
\(\Rightarrow\Sigma n_{O_2}=0,15\left(mol\right)\) \(\Rightarrow V_{O_2}=0,15\cdot22,4=3,36\left(l\right)\)

\(n_{O_2}=\dfrac{5.6}{22.4}=0.25\left(mol\right)\)
\(n_{H_2}=\dfrac{10.08}{22.4}=0.45\left(mol\right)\)
\(n_{Fe}=a\left(mol\right),n_{Al}=b\left(mol\right)\)
\(3Fe+2O_2\underrightarrow{^{^{t^0}}}Fe_3O_4\)
\(a.......\dfrac{2a}{3}\)
\(4Al+3O_2\underrightarrow{^{^{t^0}}}2Al_2O_3\)
\(b.......\dfrac{3b}{4}\)
\(n_{O_2}=\dfrac{2a}{3}+\dfrac{3b}{4}=0.25\left(mol\right)\left(1\right)\)
\(Fe+2HCl\rightarrow FeCl_2+H_2\)
\(2Al+6HCl\rightarrow2AlCl_3+3H_2\)
\(n_{H_2}=a+1.5b=0.45\left(mol\right)\left(2\right)\)
\(\left(1\right),\left(2\right):a=0.15,b=0.2\)
\(m_{Fe}=0.15\cdot56=8.4\left(g\right)\)
\(m_{Al}=0.2\cdot27=5.4\left(g\right)\)
\(\%m_{Fe}=\dfrac{8.4}{8.4+5.4}\cdot100\%=60.8\%\)
\(\%m_{Al}=100-60.8=39.2\%\)

\(n_{H_2}=\dfrac{0,953m}{22,4}=0,042545m\left(mol\right)\\ Đặt:n_{Mg}=x\left(mol\right);n_{Al}=y\left(mol\right);n_{Cu}=z\left(mol\right)\left(x,y,z>0\right)\\\Rightarrow \left\{{}\begin{matrix}24x+27y+64z=m\\40x+51y+80z=1,72m\\x+1,5y=0,042545m\end{matrix}\right.\\ \Leftrightarrow\left\{{}\begin{matrix}x\approx0,012845m\\y\approx0,0198m\\z\approx0,002455m\end{matrix}\right.\\ \Rightarrow\%m_{Cu}\approx\dfrac{0,002455.64m}{m}.100\%\approx15,712\%\\ \%m_{Al}\approx\dfrac{27.0,0198m}{m}.100\%\approx53,46\%\\ \%m_{Mg}\approx\dfrac{0,012845.24m}{m}.100\%\approx30,828\%\)

\(\left\{Mg;Al\right\}+0,125\) mol \(O2\rightarrow9,1\) gam hỗn hợp oxit.
Bảo toàn khối lượng có:
m =\(9,1-0,125\times32=5,1gam\)

\(2Mg+O_2\xrightarrow{t^o}2MgO\\ 4Al+3O_2\xrightarrow{t^o}2Al_2O_3\\ \Rightarrow \begin{cases} 24.n_{Mg}+27.n_{Al}=5,1\\ 0,5.n_{Mg}+0,75.n_{Al}=n_{O_2}=\dfrac{2,8}{22,4}=0,125 \end{cases}\\ \Rightarrow \begin{cases} n_{Mg}=0,1(mol)\\ n_{Al_2O_3}=0,1(mol) \end{cases}\\ \Rightarrow \%m_{Mg}=\dfrac{0,1.24}{5,1}.100\%\approx 47,06\%\)

Theo đề bài, ta có: \(\dfrac{m_{Mg}}{3}=\dfrac{m_{Al}}{2}\) và \(m_{Mg}+m_{Al}=6,3\)
Áp dụng tính chất dãy tỉ số bằng nhau, ta có:
\(\dfrac{m_{Mg}}{3}=\dfrac{m_{Al}}{2}=\dfrac{m_{Mg}+m_{Al}}{3+2}=\dfrac{6,3}{5}=1,26\\ \Rightarrow\left\{{}\begin{matrix}m_{Mg}=3.1,26=3,78\left(g\right)\\m_{Al}=2.1,26=2,52\left(g\right)\end{matrix}\right.\\ \Rightarrow\left\{{}\begin{matrix}n_{Mg}=\dfrac{3,78}{24}=0,1575\left(mol\right)\\n_{Al}=\dfrac{2,52}{27}=\dfrac{7}{75}\left(mol\right)\end{matrix}\right.\)
\(PTHH:2Mg+O_2\underrightarrow{t^o}2MgO\\ \left(mol\right)0,1575\rightarrow.......0,1575\\ PTHH:4Al+3O_2\underrightarrow{t^o}2Al_2O_3\\ \left(mol\right)....\dfrac{7}{75}\rightarrow.........\dfrac{7}{150}\)
Bạn dựa theo đề làm tiếp nhé, tại không thấy cái đề :v

\(\left\{{}\begin{matrix}m_{Mg}=\dfrac{40.9}{100}=3,6\left(g\right)\\m_{Al}=9-3,6=5,4\left(g\right)\end{matrix}\right.\\ \rightarrow\left\{{}\begin{matrix}n_{Mg}=\dfrac{3,6}{24}=0,15\left(mol\right)\\n_{Al}=\dfrac{5,4}{27}=0,2\left(mol\right)\end{matrix}\right.\)
PTHH:
Mg + 2HCl ---> MgCl2 + H2
0,15 ------------------------> 0,15
2Al + 6HCl ---> 2AlCl3 + 3H2
0,2 ---------------------------> 0,3
\(\rightarrow V_{H_2}=\left(0,15+0,3\right).22,4=10,08\left(l\right)\)
\(n_{H_2O}=\dfrac{6,48}{18}=0,36\left(mol\right)\)
PTHH: FexOy + yH2 --to--> xFe + yH2O
Theo pthh: \(n_{O\left(oxit\right)}=n_{H_2\left(pư\right)}=n_{H_2O}=0,36\left(mol\right)\)
\(\rightarrow m_{oxit}=15,12+16.0,36=20,88\left(g\right)\)
\(n_{Fe}=\dfrac{15,12}{56}=0,27\left(mol\right)\)
CTHH: FexOy
=> x : y = 0,27 : 0,36 = 3 : 4
=> CTHH: Fe3O4 (oxit sắt từ)
mMg = 40%x9 = 3,6(g) =>nMg=3,6:24 = 0,15 (mol)
=> mAl = 9-3,6 = 5,4(g) => nAl = 5,4:27 = 0,2 (mol)
pthh : 2Al+6HCl -> 2AlCl3+3H2
0,2 0,3
Mg+2HCl -> MgCl2 +H2
0,15 0,15
=> nH2 = 0,15 + 0,3 = 0,45 (mol)
=> VH2 = 0,45.22,4 = 10,08 (L)
mH2 = 0,45 . 2 = 0,9 (mol)
áp dụng BLBTKL ta có :
mH2 + moxit sắt = mFe + mH2O
=> moxit sắt = 20,7 (g)

Gọi \(\left\{{}\begin{matrix}n_{Al}=x\left(mol\right)\\n_{Fe}=y\left(mol\right)\end{matrix}\right.\)
\(n_{H_2}=\dfrac{4,48}{22,4}=0,2mol\)
\(2Al+6HCl\rightarrow2AlCl_3+3H_2\)
\(Fe+2HCl\rightarrow FeCl_2+H_2\)
Theo pt: \(\Rightarrow\left\{{}\begin{matrix}3x+y=0,2\\27x+56y=5,5\end{matrix}\right.\Rightarrow\left\{{}\begin{matrix}x=\dfrac{19}{470}\\y=\dfrac{37}{470}\end{matrix}\right.\)
\(\%m_{Al}=\dfrac{\dfrac{19}{470}\cdot27}{5,5}\cdot100\%=19,84\%\)
\(\%m_{Fe}=100\%-19,84\%=80,16\%\)